-
Name
4'-Methoxyacetophenone
- EINECS 202-815-9
- CAS No. 100-06-1
- Article Data1225
- CAS DataBase
- Density 1.035 g/cm3
- Solubility Insoluble in water
- Melting Point 36-38 °C(lit.)
- Formula C9H10O2
- Boiling Point 256.4 °C at 760 mmHg
- Molecular Weight 150.177
- Flash Point 113.2 °C
- Transport Information
- Appearance White crystals or crystalline powder
- Safety 37-37/39-26-36
- Risk Codes 22-38-36/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Acetophenone,4'-methoxy- (8CI);Benzophenone, p-methoxy- (3CI);1-(4-Methoxyphenyl)ethanone;1-Acetyl-4-methoxybenzene;4-Acetylanisole;4-Methoxyphenyl methyl ketone;Acetoanisole;Linarodin;Methyl 4-methoxyphenyl ketone;Methyl p-methoxyphenyl ketone;Novatone;Vananote;p-Acetylanisole;p-Anisyl methyl ketone;p-Methoxy(acetyl)benzene;p-Methoxyacetophenone;p-Methoxyphenyl methyl ketone;para-Methoxyacetophenone;
- PSA 26.30000
- LogP 1.89780
Synthetic route

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; C21H19N5Pd(2+)*2BF4(1-) In decane; acetonitrile at 45℃; for 4h; Wacker Oxidation; | 100% |
| With water; oxygen In methanol; dimethyl sulfoxide at 80℃; under 1520.1 Torr; for 20h; Wacker Oxidation; Autoclave; | 99% |
| With palladium diacetate; 9-tert-butyl-10-methylanthracene ozonide In acetonitrile for 72h; Ambient temperature; | 93% |

| Conditions | Yield |
|---|---|
| With 2.9-dimethyl-1,10-phenanthroline; oxygen; sodium hydrogencarbonate; gold(I) chloride In water at 100℃; under 38002.6 Torr; for 24h; | 100% |
| With Cp*Ir(6,6'-dionato-2,2'-bipyridine)(H2O) In pentane for 5h; Reflux; | 100% |
| With diisopropoxyaluminium trifluoroacetate; 4-nitrobenzaldehdye In benzene for 0.25h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| With polystyrene-bound tetrafluorophenylbis(triflyl)methane In nitromethane at 50℃; for 2h; Friedel-Crafts acylation; | 100% |
| With lithium perchlorate at 60℃; for 1h; | 100% |
| With Sulfate; zirconium(IV) oxide at 110℃; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: acetic acid; methoxybenzene With trifluoroacetic anhydride In dichloromethane at 20℃; for 0.25h; Stage #2: With trifluorormethanesulfonic acid In dichloromethane at 20℃; for 1h; | 100% |
| With methanesulfonic acid; pyrographite at 80℃; for 0.333333h; Friedel-Crafts acylation; | 98% |
| With aluminum oxide; trifluoroacetic anhydride for 0.166667h; Ambient temperature; | 96% |
-
-
128881-90-3
1-(1,1-Bis-ethylsulfanyl-ethyl)-4-methoxy-benzene

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With gallium(III) trichloride In dichloromethane; water for 0.25h; Ambient temperature; | 100% |
-
-
100-66-3
methoxybenzene

-
-
79562-11-1
N,N-diacetyl-p-nitrophenylsulphenamide

-
A
-
22865-50-5
4-(4-methoxyphenylsulfanyl)nitrobenzene

-
B
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 190 - 200℃; | A 100% B 21% |


| Conditions | Yield |
|---|---|
| With gold(III) tribromide; water at 200℃; for 0.333333h; microwave irradiation; | 100% |
| With Au nanoparticles covalently bonded to HS/SO3H functionalized periodic mesoporous organosilica (Et) at 70℃; for 1.5h; neat (no solvent); | 100% |
| With hydrogenchloride; tetrakis(triphenylphosphine) palladium(0) In 1,4-dioxane at 48℃; for 20h; Solvent; Schlenk technique; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With AcrH2 In acetonitrile at 298℃; for 19h; Irradiation; | 100% |
| With AcrH2 In acetonitrile at 298℃; for 19h; Irradiation; | 100% |
| With decaborane; palladium on activated charcoal In methanol at 20℃; for 2h; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Heating; | 100% |
| With potassium hydroxide; 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate at 25℃; for 2h; Williamson synthesis; | 99% |
| With potassium carbonate In acetone Heating; | 98% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; Tonsil In ethyl acetate for 1.5h; Heating; | 100% |
| With CuCl2*2H2O for 0.00277778h; microwave irradiation; | 95% |
| With benzyltriphenylphosphonium dichromate; silica gel for 0.25h; | 95% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; 1-ethyl-3-methyl-1H-imidazol-3-ium chloride at -10℃; for 0.25h; | 99% |
| With zinc at 80 - 82℃; for 0.00194444h; Friedel-Crafts acylation; microwave irradiation; | 99% |
| With indium(III) tosylate In dodecane; nitromethane for 1h; Friedel-Crafts Acylation; Schlenk technique; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxyacetophenone oxime With hexachlorodisilane; silica gel In toluene at 110℃; for 0.5h; Stage #2: With water In toluene for 0.5h; | 99% |
| With N,N'-dibromo-N,N'-(1,2-ethanediyl)bis(p-toluenesulfonamide) In tetrachloromethane at 20℃; for 2h; Product distribution; | 97% |
| With formaldehyd; sodium dodecyl-sulfate In water at 50℃; for 2h; Ultrasound irradiation; | 97% |

| Conditions | Yield |
|---|---|
| With oxygen In dimethyl sulfoxide at 20℃; under 760.051 Torr; for 6h; Irradiation; | 99% |
| With tert.-butylhydroperoxide In chlorobenzene at 99.84℃; for 10h; | 98% |
| With dihydroxy-methyl-borane; bathophenanthroline; copper (I) acetate; lithium carbonate; N-fluorobis(benzenesulfon)imide In chlorobenzene at 45℃; for 16h; | 96% |
-
-
36881-00-2
2-(4-methoxyphenyl)-2-methyI-1,3-dioxolane

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With cerium triflate In nitromethane; water at 20℃; for 6h; | 99% |
| With water at 120℃; for 0.5h; microwave irradiation; | 99% |
| With Montmorillonite K 10; water In acetone for 0.5h; Heating; | 97% |
-
-
19513-78-1
1-(4-methoxy-phenyl)-2-phenoxy-ethanone

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With ammonium chloride; zinc In ethanol for 0.5h; Heating; | 99% |
| With 2,6-bis[1-(2,6-dimethylphenylimino)ethyl]pyridine cobalt(II)dichloride; bis(pinacol)diborane; sodium t-butanolate In tetrahydrofuran; methanol at 65℃; for 3h; Schlenk technique; Inert atmosphere; | 92% |
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate / tetrahydrofuran; water / 2 h / 20 °C 2: sodium salt of dibutyl phosphate; Methyl thioglycolate; [Ir(ppy)2(dtbpy)]PF6 / N,N-dimethyl acetamide / 24 h / 25 °C / Irradiation; Sealed tube; Inert atmosphere View Scheme |
-
-
19513-80-5
2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)ethan-1-one

-
A
-
90-05-1
2-methoxy-phenol

-
B
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With formic acid; N-ethyl-N,N-diisopropylamine; Lumogen F Orange 240 In acetonitrile at 25℃; for 8h; Reagent/catalyst; UV-irradiation; Inert atmosphere; | A 99% B 99% |
| With formic acid; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; N-ethyl-N,N-diisopropylamine In ethanol at 20℃; Irradiation; chemoselective reaction; | A 95% B 72% |
| With formic acid; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 12h; Reagent/catalyst; Irradiation; chemoselective reaction; | A 89% B 88% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; palladium on activated charcoal In water; ethyl acetate at 80℃; for 1h; | A 95% B 99% |
| With 5%-palladium/activated carbon; ammonium formate In water at 80℃; for 1h; | A n/a B 98% |
| With C32H25Cl2N6O2Rh2(1+)*Cl(1-); sodium hydroxide In water at 110℃; for 18h; Inert atmosphere; | A 89% B 86% |
-
-
2040-01-9
2',3',4',5',6'-pentamethylacetophenone

-
-
100-66-3
methoxybenzene

-
A
-
579-74-8
2-Methoxyacetophenone

-
B
-
700-12-9
pentamethylbenzene,

-
C
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| trifluoroacetic acid for 20h; Heating; | A n/a B 98% C n/a |
| With sodium 2,2,2-trifluoroacetate; trifluoroacetic acid for 0.5h; Product distribution; Rate constant; Heating; with various additives and additive amounts; | A n/a B 38% C n/a |

-
-
24310-46-1
4-methoxyacetophenone phenylhydrazone

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With benzyltriphenylphosphonium dichromate; silica gel for 0.116667h; | 98% |
| With 1-benzyl-1-aza-4-azoniabicyclo<2.2.2>octane periodate In acetonitrile for 0.75h; Oxidation; Heating; | 97% |
| With Oxone; water; potassium hydrogencarbonate In acetone for 0.5h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With 1-benzyl-4-aza-1-azoniabiyclo<2.2.2>octane peroxodisulfate In acetonitrile for 0.916667h; Heating; | 98% |
| With Oxone; water; potassium hydrogencarbonate In acetonitrile for 0.5h; Heating; | 98% |
| With benzyltriphenylphosphonium dichromate In acetonitrile for 0.583333h; Oxidation; Heating; | 98% |
-
-
108-24-7
acetic anhydride

-
-
100-66-3
methoxybenzene

-
A
-
64-19-7
acetic acid

-
B
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With lanthanum(lll) triflate In nitromethane at 50℃; for 24h; Kinetics; Product distribution; Further Variations:; Temperatures; Solvents; Reagents; Friedel-Crafts acylation; | A n/a B 98% |
| Ce-Clay at 100℃; for 1 - 5h; | A n/a B 35% |
| La-Clay at 100℃; for 1 - 5h; | A n/a B 15% |

-
-
29509-29-3
1-(4-methoxyphenyl)-2-phenoxyethan-1-ol

-
A
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
B
-
108-95-2
phenol

| Conditions | Yield |
|---|---|
| With carbonyl bis(hydrido)tris(triphenylphosphine)ruthenium(II); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In toluene at 135℃; for 4h; Inert atmosphere; | A 98% B n/a |
| With sodium tetrahydroborate; palladium on activated charcoal In water; ethyl acetate at 80℃; for 1h; | A 98% B n/a |
| With [Ir(ppy)2(dtbpy)]PF6; sodium salt of dibutyl phosphate; Methyl thioglycolate In N,N-dimethyl acetamide at 25℃; for 24h; Reagent/catalyst; Solvent; Irradiation; Sealed tube; Inert atmosphere; | A 86% B 71% |
-
-
19513-80-5
2-(2-methoxyphenoxy)-1-(4-methoxyphenyl)ethan-1-one

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; ammonium formate In ethanol; water at 80℃; for 1h; | 98% |
| With 2,6-bis[1-(2,6-dimethylphenylimino)ethyl]pyridine cobalt(II)dichloride; bis(pinacol)diborane; sodium t-butanolate In tetrahydrofuran; methanol at 65℃; for 3h; Schlenk technique; Inert atmosphere; | 89% |
| With formic acid; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; N-ethyl-N,N-diisopropylamine In ethanol at 20℃; for 12h; Irradiation; chemoselective reaction; |
-
-
29509-29-3
1-(4-methoxyphenyl)-2-phenoxyethan-1-ol

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; ammonium formate In tert-butyl methyl ether; water at 80℃; for 3h; | 98% |
-
-
296278-03-0
1-(4-methoxyphenyl)-2-(naphthalen-2-yloxy)ethan-1-one

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With 2,6-bis[1-(2,6-dimethylphenylimino)ethyl]pyridine cobalt(II)dichloride; bis(pinacol)diborane; sodium t-butanolate In tetrahydrofuran; methanol at 65℃; for 3h; Schlenk technique; Inert atmosphere; | 98% |
-
-
637-69-4
4-Methoxystyrene

-
A
-
3319-15-1
rac-1-(4-methoxyphenyl)-ethanol

-
B
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With 2.9-dimethyl-1,10-phenanthroline; phenylsilane; iron(II) chloride In ethanol at 20℃; for 4.5h; Reagent/catalyst; Wacker Oxidation; | A 2% B 97% |
| With triethylsilane; (1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-Hexadecafluorophthalocyaninato)iron(II); oxygen In ethanol at 20℃; under 760.051 Torr; for 9.5h; Wacker Oxidation; Sealed tube; Green chemistry; chemoselective reaction; | A 12% B 85% |
| With sodium hydroxide; sodium tetrahydroborate; oxygen; 5,10,15,20-tetrakis(1-methyl-4-pyridino)porphyrine tertachloride In water Ambient temperature; pH 12; | A 14% B 65% |
| With triethylsilane; [5,10,15,20-tetra(2,6-dichlorophenyl)porphyrinato]cobalt(II); oxygen; phosphorous acid trimethyl ester 1.) 2-propanol, dichloromethane, 28 deg C, 1 atm, 30 min, 2.) 2-propanol, dichloromethane, RT, 2 h; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
3319-15-1
rac-1-(4-methoxyphenyl)-ethanol

-
-
74087-85-7
3,3-dimethyldioxirane

-
A
-
4136-21-4
p-methoxybenzoylmethanol

-
B
-
100-06-1
1-(4-methoxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| In acetone at 25℃; for 3h; | A 3% B 97% |
| In acetone at 25℃; for 3h; Thermodynamic data; Rate constant; Ea, ΔH(excit.), ΔS(excit.), ΔG(excit.); | A 3% B 97% |


| Conditions | Yield |
|---|---|
| With potassium chloride; acetic anhydride In nitromethane; toluene | 97% |


| Conditions | Yield |
|---|---|
| With 4-phenylnaphthalene-1,2-dione In acetonitrile at 80℃; for 36h; | 97% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; laccasefrom Trametes versicolor; oxygen In water at 30℃; pH=4.5; Enzymatic reaction; | 84% |
| With caesium carbonate; dibenzoyl peroxide In N,N-dimethyl-formamide at 0 - 50℃; | 69% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 0.025h; Irradiation; | 100% |
| With 4 A molecular sieve; NAP-MgO In toluene for 16h; Claisen-Schmidt condensation; Heating; | 98% |
| With sodium hydroxide In ethanol at 20℃; Cooling with ice; | 95% |
-
-
123-11-5
4-methoxy-benzaldehyde

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
41564-67-4
(E)-1,3-bis(4-methoxyphenyl)-2-propene-1-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol at 20℃; for 20h; Aldol Condensation; | 100% |
| With sodium hydroxide In ethanol at 20℃; for 48h; | 99% |
| With sodium hydroxide In ethanol at 20℃; for 48h; Claisen-Schmidt Condensation; | 98% |
-
-
95-92-1
oxalic acid diethyl ester

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
35322-20-4
ethyl 3-(4-methoxybenzoyl)pyruvate

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-methoxyphenyl)ethanone With sodium In ethanol at 20℃; for 0.5h; Stage #2: oxalic acid diethyl ester at 20 - 80℃; for 3h; | 100% |
| With sodium hydride In N,N-dimethyl-formamide at 0 - 100℃; | 100% |
| Stage #1: oxalic acid diethyl ester With sodium methylate In diethyl ether at 20℃; Inert atmosphere; Stage #2: 1-(4-methoxyphenyl)ethanone In diethyl ether at 20℃; for 12h; | 92% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 0℃; Inert atmosphere; | 100% |
| Stage #1: 1-(4-methoxyphenyl)ethanone With C24H20Cl2F5NRuS; isopropyl alcohol at 82℃; for 0.166667h; Stage #2: With potassium hydroxide at 82℃; for 1h; | 100% |
| With hydrogen; Ru(1,3-dimesityl-2,3-dihydro-1H-imidazol-2-yl)(PPh3)2CO(H)2 In benzene at 70℃; under 3800 Torr; for 24h; | 99% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride | 100% |
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water at 95℃; | 100% |
| With pyridine; hydroxylamine hydrochloride In ethanol at 75℃; for 22h; | 99% |

| Conditions | Yield |
|---|---|
| With selenium (IV) oxide In 1,4-dioxane; water Heating; | 100% |
| With tris(2,2'-bipyridyl)ruthenium dichloride; dioxane dibromide; sodium L-ascorbate In acetonitrile at 20℃; for 8h; Irradiation; Green chemistry; | 91% |
| With selenium(IV) oxide In 1,4-dioxane for 3h; Reflux; | 88% |

| Conditions | Yield |
|---|---|
| With bromine In 1,4-dioxane; diethyl ether for 0.5h; Ambient temperature; | 100% |
| With N-Bromosuccinimide; toluene-4-sulfonic acid In chloroform at 20℃; for 12h; | 98.7% |
| With bis(dimethylacetamide)hydrogen tribromide In methanol at 20 - 45℃; for 0.25h; | 96% |

| Conditions | Yield |
|---|---|
| With hydrogen under 2250.23 Torr; for 4h; | 100% |
| With palladium dichloride In methanol at 40℃; for 24h; Inert atmosphere; Green chemistry; chemoselective reaction; | 99% |
| With hydrogen In 1,4-dioxane at 200℃; under 15001.5 Torr; | 98.9% |

| Conditions | Yield |
|---|---|
| With perchloric acid for 0.666667h; | 100% |
-
-
616-38-6
carbonic acid dimethyl ester

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
22027-50-5
methyl 3-(4-methoxybenzoyl)acetate

| Conditions | Yield |
|---|---|
| With sodium hydride In toluene at 110℃; | 100% |
| Stage #1: carbonic acid dimethyl ester With sodium hydride In toluene at 110℃; Stage #2: 1-(4-methoxyphenyl)ethanone In toluene at 110℃; | 100% |
| With sodium hydride In toluene for 4h; Inert atmosphere; Reflux; | 98% |
-
-
33868-76-7
1-(4-methoxyphenyl)-3,3-bis(methylsulfanyl)propenone

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
78227-65-3
(E)-1,5-Bis-(4-methoxy-phenyl)-3-methylsulfanyl-pent-2-ene-1,5-dione

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran for 3h; Ambient temperature; | 100% |
-
-
33868-76-7
1-(4-methoxyphenyl)-3,3-bis(methylsulfanyl)propenone

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
78227-65-3
(E)-1,5-Bis-(4-methoxy-phenyl)-3-methylsulfanyl-pent-2-ene-1,5-dione

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran Ambient temperature; | 100% |
-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
18212-22-1
4-(4-methoxy-phenyl)-[1,2,3]thiadiazole

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-methoxyphenyl)ethanone With polystyrene-sulfonylhydrazide resin; acetic acid In tetrahydrofuran at 50℃; for 4h; Solid phase reaction; Stage #2: With thionyl chloride In 1,2-dichloro-ethane at 60℃; for 5h; Solid phase reaction; Hurd-Mori cyclization; | 100% |
| With iodine; potassium thioacyanate; toluene-4-sulfonic acid hydrazide; copper dichloride In dimethyl sulfoxide at 100℃; for 1h; Sealed tube; | 81% |
| With iodine; potassium thioacyanate; toluene-4-sulfonic acid hydrazide; copper(l) chloride In dimethyl sulfoxide at 130℃; for 1h; | 81% |

| Conditions | Yield |
|---|---|
| With Mg10Al2(OH)24CO3; oxygen; benzaldehyde In 1,2-dichloro-ethane at 40℃; for 24h; | 100% |
| With bis-trimethylsilanyl peroxide; 4 A molecular sieve; tin(IV) chloride; rac-diaminocyclohexane In tetrahydrofuran; dichloromethane for 3h; Ambient temperature; | 97% |
| With 3-chloro-benzenecarboperoxoic acid; scandium tris(trifluoromethanesulfonate) In dichloromethane for 1h; Ambient temperature; | 95% |
-
-
139343-81-0
(S(S)R)-(+)-3-methyl-2-pivaloyl-2,3-dihydroisothiazole 1-oxide

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
174736-11-9
N-((R)-1-{2-[(S)-2-(4-Methoxy-phenyl)-2-oxo-ethanesulfinyl]-phenyl}-ethyl)-2,2-dimethyl-propionamide

| Conditions | Yield |
|---|---|
| With sodium hexamethyldisilazane In tetrahydrofuran; toluene 1.) -78 deg C, 1 h, 2.) -78 deg C, 1 h; -78 deg C to r.t.; | 100% |
-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
1517-70-0, 1572-97-0, 3319-15-1, 43230-31-5
(S)-1-(4-Methoxyphenyl)ethanol

| Conditions | Yield |
|---|---|
| With dimethylsulfide borane complex; 4-chloro-2-(((1S,2R)-1-hydroxy-1-phenylpropan-2-ylamino)methyl)phenol In tetrahydrofuran for 2h; Inert atmosphere; Reflux; optical yield given as %ee; enantioselective reaction; | 100% |
| diethylzinc; (+)-N,N'-Bis<(R)-1-phenylethyl>-1,2-ethylendiamin | 99% |
| With (mer-[(S,S)-1,5-dimethyl-2,4-bis(4-phenyl-1,3-oxazolin-2-yl)benzene(1-)]Ru(CO)Cl)2(ZnCl2); hydrogen; sodium methylate; (-)-(S)-1-Anthracen-9-ylethanol In isopropyl alcohol at 40℃; under 22801.5 Torr; for 24h; Inert atmosphere; Autoclave; optical yield given as %ee; enantioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With (S,S)-RuCl2(2,2'-bis(di-3,5-xylylphosphino)-1,1'-binaphthyl)(1,1-dianisyl-2-isopropyl-1,2-ethylenediamine); potassium tert-butylate; hydrogen In isopropyl alcohol at 26 - 30℃; under 7600 Torr; for 1h; | 100% |
| With Trimethyl borate; (S)-diphenylprolinol; dimethylsulfide borane complex In tetrahydrofuran; toluene at 25℃; | 99% |
| With potassium tert-butylate; hydrogen; [bis(2-methylallyl)cycloocta-1,5-diene]ruthenium(II); (R)-Ph-BINAN-H-Py In isopropyl alcohol at 25℃; under 38000 Torr; for 15h; | 99.3% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate In methanol Heating; | 100% |
| With hydroxylamine hydrochloride; sodium acetate In methanol; water Reflux; | 100% |
| With hydroxylamine hydrochloride; sodium acetate In methanol Reflux; | 100% |
-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
18096-70-3
3-(dimethylamino)-1-(4-methoxyphenyl)prop-2-en-1-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 3.5h; Reflux; | 100% |
| In N,N-dimethyl-formamide for 24h; Reflux; | 98% |
| In neat (no solvent) at 160℃; for 0.25h; Microwave irradiation; | 95% |
-
-
50-00-0
formaldehyd

-
-
31252-42-3
4-benzylpyperidine

-
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
-
1037020-22-6
C35H44N2O2

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol Mannich reaction; Heating; | 100% |

4'-Methoxyacetophenone Consensus Reports
Reported in EPA TSCA Inventory.
4'-Methoxyacetophenone Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid
4'-Methoxyacetophenone Specification
The 4'-Methoxyacetophenone with CAS registry number of 100-06-1 is also known as 4-Acetylanisole. The IUPAC name is 1-(4-Methoxyphenyl)ethanone. It belongs to product categories of Acetophenone Series; FINE Chemical & INTERMEDIATES; Aromatic Acetophenones & Derivatives (substituted); Acetophenones (Building Blocks for Liquid Crystals); Building Blocks for Liquid Crystals; Functional Materials. Its EINECS registry number is 202-815-9. In addition, the formula is C9H10O2 and the molecular weight is 150.19. This chemical is a white crystals or crystalline powder and should be sealed in ventilated, cool place away from fire, heat. What's more, this chemical is used for the preparation of flavor and it can also be used in organic synthesis.
Physical properties about 4'-Methoxyacetophenone are: (1)ACD/LogP: 1.74; (2)ACD/LogD (pH 5.5): 1.74; (3)ACD/LogD (pH 7.4): 1.74; (4)ACD/BCF (pH 5.5): 12.37; (5)ACD/BCF (pH 7.4): 12.37; (6)ACD/KOC (pH 5.5): 210.65; (7)ACD/KOC (pH 7.4): 210.65; (8)#H bond acceptors: 2; (9)#Freely Rotating Bonds: 2; (10)Index of Refraction: 1.504; (11)Molar Refractivity: 42.95 cm3; (12)Molar Volume: 144.9 cm3; (13)Surface Tension: 33.6 dyne/cm; (14)Density: 1.035 g/cm3; (15)Flash Point: 113.2 °C; (16)Enthalpy of Vaporization: 49.39 kJ/mol; (17)Boiling Point: 256.4 °C at 760 mmHg; (18)Vapour Pressure: 0.0155 mmHg at 25 °C.
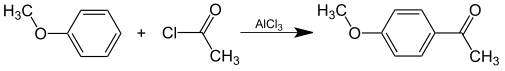
Preparation of 4'-Methoxyacetophenone: it is prepared synthetically by Friedel-Crafts acylation of anisole with acetyl chloride.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes and skin and even harmful by inhalation, in contact with skin and if swallowed. During using it, wear suitable protective clothing, gloves and eye/face protection. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CC(=O)C1=CC=C(C=C1)OC
2. InChI: InChI=1S/C9H10O2/c1-7(10)8-3-5-9(11-2)6-4-8/h3-6H,1-2H3
3. InChIKey: NTPLXRHDUXRPNE-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| human | TCLo | inhalation | 1700ug/m3/39W (1.7mg/m3) | CARDIAC: PULSE RATE INCREASE WITHOUT FALL IN BP VASCULAR: BP ELEVATION NOT CHARACTERIZED IN AUTONOMIC SECTION | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 50(4), Pg. 86, 1985. |
| mouse | LD50 | oral | 820mg/kg (820mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: IRRITABILITY BEHAVIORAL: MUSCLE WEAKNESS | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 50(4), Pg. 86, 1985. |
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 12, Pg. 927, 1974. | |
| rat | LD50 | oral | 1720mg/kg (1720mg/kg) | Food and Cosmetics Toxicology. Vol. 12, Pg. 927, 1974. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View