-
Name
4-AMINOSTYRENE
- EINECS 216-185-8
- CAS No. 1520-21-4
- Article Data154
- CAS DataBase
- Density 1.012 g/cm3
- Solubility
- Melting Point 23 °C
- Formula C8H9N
- Boiling Point 238.8 °C at 760 mmHg
- Molecular Weight 119.166
- Flash Point 108.6 °C
- Transport Information
- Appearance liquid
- Safety 26-36/37
- Risk Codes 36/37/38-48/20/21/22-43-40-33
-
Molecular Structure
-
Hazard Symbols
 Xn;
Xn;  T;
T;  Xi
Xi
- Synonyms Aniline,p-vinyl- (6CI,7CI,8CI);4-Ethenylbenzenamine;4-Vinylaniline;4-Vinylbenzenamine;4-Vinylphenylamine;p-Aminostyrene;p-Ethenylaniline;p-Vinylaniline;
- PSA 26.02000
- LogP 2.49300
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In water for 0.233333h; Kinetics; Catalytic behavior; Reagent/catalyst; | 100% |
| With hydrazine hydrate In ethanol at 40℃; for 0.5h; | 99% |
| With hydrogen; silver In dodecane at 110℃; under 4500.45 Torr; for 6h; | 98% |
-
-
14235-81-5
4-Ethynylaniline

-
-
1520-21-4
4-vinyl benzylamine

| Conditions | Yield |
|---|---|
| With hydrogen In toluene at 20℃; under 22801.5 Torr; for 12h; Autoclave; chemoselective reaction; | 99% |
| With hydrogen; 3-azapentane-1,5-diamine In methanol at 25℃; for 6h; | 90% |
| With Au0998Ag0002; hydrogen; diethylamine at 90℃; under 6080.41 Torr; for 24h; chemoselective reaction; | 76% |

| Conditions | Yield |
|---|---|
| With potassium fluoride; Pd (0.87 wt%)/MgO In N,N-dimethyl-formamide at 130℃; under 1500.15 Torr; for 5h; Reagent/catalyst; Time; Hiyama Coupling; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With hydrogen In hexane; toluene at 30℃; under 760.051 Torr; for 8h; | A 95% B 5% |
| With hydrogen In 1,4-dioxane at 20℃; for 24h; chemoselective reaction; | |
| With hydrogen In 1,4-dioxane at 85℃; under 4500.45 Torr; for 14h; chemoselective reaction; | A 24 %Chromat. B n/a |
| With carbon monoxide; hydrogen In tetrahydrofuran at 70℃; under 7757.43 Torr; for 6h; Autoclave; |
-
-
42254-91-1
4,4'-divinylazobenzene

-
-
1520-21-4
4-vinyl benzylamine

| Conditions | Yield |
|---|---|
| With aminomethylpolystyrene-supported formate; palladium on activated charcoal In methanol at 20℃; for 4h; | 94% |
| With polystyrene-CH2-NH3(+)HCO2(-); magnesium In methanol at 20℃; for 0.333333h; | 93% |
| With zinc In methanol at 25℃; for 0.166667h; Inert atmosphere; | 93% |
| With aminomethyl polystyrene resin formic acid salt; zinc In methanol at 20℃; for 0.3h; | 92% |
| With magnesium In methanol at 25℃; for 0.2h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With borane-ammonia complex In ethanol at 20℃; for 0.25h; Reagent/catalyst; Schlenk technique; | A 91.53% B 8.47% |
| With borane-ammonia complex In methanol at 24.84℃; for 2h; Schlenk technique; Inert atmosphere; Irradiation; | A 71% B 29% |
| With hydrogenchloride; iron In water for 3h; Reflux; Overall yield = 0.25 g; |
-
-
1112-55-6
tetravinylsilane

-
-
540-37-4
p-aminoiodobenzene

-
A
-
1520-21-4
4-vinyl benzylamine

-
B
-
621-96-5
4,4'-diaminostilbene

| Conditions | Yield |
|---|---|
| With potassium fluoride; Pd (1.04 wt%)/TiO2 In N,N-dimethyl-formamide at 130℃; under 1500.15 Torr; for 10h; Hiyama Coupling; Inert atmosphere; | A 91% B 6% |


| Conditions | Yield |
|---|---|
| With bis(η3-allyl-μ-chloropalladium(II)); tris(diethylamino)sulfonium difluorotrimethylsiliconate In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide at 50℃; for 2h; | 85% |
| With potassium fluoride; Pd (0.87 wt%)/MgO In N,N-dimethyl-formamide at 130℃; under 1500.15 Torr; for 9h; Hiyama Coupling; Inert atmosphere; | 73% |
-
-
100-13-0
4-nitrostyrene

-
A
-
100-12-9
4-ethylnitrobenzene

-
B
-
1520-21-4
4-vinyl benzylamine

-
C
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| With borane-ammonia complex In ethanol at 20℃; for 0.25h; Reagent/catalyst; Schlenk technique; | A 10.86% B 76.84% C 12.25% |
| With 4-methylcyclohexene In methanol at 69.84℃; for 1h; Inert atmosphere; | |
| With platinum doped titanium oxide In toluene at 80℃; under 7500.75 Torr; Autoclave; |


-
-
1520-21-4
4-vinyl benzylamine

| Conditions | Yield |
|---|---|
| Stage #1: 4-vinylazidobenzene With hydrazine hydrate for 0.166667h; Inert atmosphere; Stage #2: for 16h; Irradiation; chemoselective reaction; | 65% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 200 - 260℃; under 10 - 14 Torr; | 64% |
| With 1,4-dioxane; potassium unter vermindertem Druck; |

| Conditions | Yield |
|---|---|
| With dicyclohexyl(2',4',6'-triisopropyl-5-methoxy-3,4,6-trimethyl-[1,1'-biphenyl]-2-yl)phosphine; C50H70NO4PPdS; C50H70NO4PPdS; dicyclohexyl(2',4',6'-triisopropyl-4-methoxy-3,5,6-trimethyl-[1,1'-biphenyl]-2-yl)phosphine; ammonia; sodium t-butanolate In 1,4-dioxane at 80℃; for 24h; Inert atmosphere; | 58% |
| With bis(η3-allyl-μ-chloropalladium(II)); 2-[di-(3S,5S,7S)-adamantan-1-ylphosphino]-N,N-dimethylaniline; ammonia; sodium t-butanolate In 1,4-dioxane at 110 - 120℃; Buchwald-Hartwig amination; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: vinyl magnesium bromide With gallium(III) trichloride In tetrahydrofuran; hexane; dimethyl sulfoxide at 25℃; Stage #2: p-aminoiodobenzene With tris-(o-tolyl)phosphine; tris(dibenzylideneacetone)dipalladium(0) chloroform complex In tetrahydrofuran; hexane; dimethyl sulfoxide Heating; | 45% |

| Conditions | Yield |
|---|---|
| With tributyl-amine; potassium carbonate; palladium In N,N-dimethyl-formamide at 150℃; under 7757.43 Torr; for 1h; Heck reaction; microwave irradiation; | 44% |
-
-
6531-13-1
1-[4-nitrophenyl]-1-ethanol

-
A
-
1520-21-4
4-vinyl benzylamine

-
B
-
99-92-3
4-Aminoacetophenone

-
C
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| With sol-gel entrapped pyridinium dichromate; hydrogen; sol-gel entrapped RhCl[P(C6H5)3]3 In 1,2-dichloro-ethane at 70℃; under 15960 Torr; for 16h; catalyst and pyridinium dichromate entrapped in separate sol-gel matrices; | A n/a B 42% C n/a |

-
-
93453-80-6, 14572-89-5
1-(4-aminophenyl)ethanol

-
-
1520-21-4
4-vinyl benzylamine

| Conditions | Yield |
|---|---|
| With potassium hydrogensulfate; boric acid; hydroquinone at 65 - 255℃; under 30 - 100 Torr; | 1.8% |
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| bei der Destillation unter vermindertem Druck; |

-
-
1520-21-4
4-vinyl benzylamine

| Conditions | Yield |
|---|---|
| With aluminum oxide at 250℃; unter vermindertem Druck; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 96 percent / methanesulfonyl chloride; 1,8-diazabicyclo[5.4.0]undec-7-ene / tetrahydrofuran / 16 h / 0 °C 2: 6.0 g / NH4Cl; Zn powder / H2O; acetone / 0.5 h / Heating View Scheme |
-
-
100-19-6
(4-nitrophenyl)ethanone

-
A
-
1520-21-4
4-vinyl benzylamine

-
B
-
99-92-3
4-Aminoacetophenone

-
C
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| With hydrogen; Au/Fe(OH)x In tetrahydrofuran at 95℃; under 7500.75 Torr; for 0.75h; Product distribution; Further Variations:; Catalysts; |

-
-
100-19-6
(4-nitrophenyl)ethanone

-
A
-
1520-21-4
4-vinyl benzylamine

-
B
-
93453-80-6, 14572-89-5
1-(4-aminophenyl)ethanol

-
C
-
99-92-3
4-Aminoacetophenone

-
D
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethanol at 25℃; for 2h; aq. buffer; | A 30 %Chromat. B 26 %Chromat. C 27 %Chromat. D 17 %Chromat. |

-
-
93453-80-6, 14572-89-5
1-(4-aminophenyl)ethanol

-
A
-
1520-21-4
4-vinyl benzylamine

-
B
-
589-16-2
4-aminoethylbenzene

| Conditions | Yield |
|---|---|
| With potassium hydrogensulfate; hydroquinone at 250 - 255℃; under 30 - 100 Torr; for 1.5h; | |
| With boric acid; hydroquinone at 245 - 255℃; under 80 Torr; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate / ethanol / 40 °C 2: potassium hydrogensulfate; boric acid; hydroquinone / 65 - 255 °C / 30 - 100 Torr View Scheme | |
| With C18H21BrMnN3O3; potassium tert-butylate; isopropyl alcohol at 40℃; for 24h; | 27 %Chromat. |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate / ethanol / 40 °C 2: boric acid; hydroquinone / 245 - 255 °C / 80 Torr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrogenchloride; tin(II) chloride hydrate / ethanol; water / 50 °C 2: sodium tetrahydroborate / ethanol / 40 °C 3: potassium hydrogensulfate; boric acid; hydroquinone / 65 - 255 °C / 30 - 100 Torr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrogenchloride; tin(II) chloride hydrate / ethanol; water / 50 °C 2: sodium tetrahydroborate / ethanol / 40 °C 3: boric acid; hydroquinone / 245 - 255 °C / 80 Torr View Scheme |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
1520-21-4
4-vinyl benzylamine

-
-
57295-14-4
tert-butyl 4-vinylphenylcarbamate

| Conditions | Yield |
|---|---|
| In water at 35℃; for 4h; | 99% |
| In dichloromethane at 20℃; for 14h; | 98% |
| With triethylamine In tetrahydrofuran at 0 - 30℃; for 111.5h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethyl acetate at 0 - 20℃; Inert atmosphere; | 99% |
| With triethylamine In ethyl acetate at 0 - 20℃; for 24h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Inert atmosphere; | 98% |
| In dichloromethane at 20℃; Inert atmosphere; | 89% |
| With pyridine In dichloromethane Acetylation; | 85% |

| Conditions | Yield |
|---|---|
| With iron(III) chloride; lithium aluminium tetrahydride; hydrogen In tetrahydrofuran at 18℃; under 7500.75 Torr; for 20h; Inert atmosphere; Sealed tube; | 97% |
| With oxygen; hydrazine hydrate In propan-1-ol at 100℃; under 15001.5 Torr; | 95% |
| With oxygen; hydrazine hydrate In propan-1-ol at 100℃; under 15001.5 Torr; for 0.166667h; Flow reactor; | 95% |
-
-
1520-21-4
4-vinyl benzylamine

| Conditions | Yield |
|---|---|
| With n-butyllithium In diethyl ether Inert atmosphere; | 97% |
| With n-butyllithium In tetrahydrofuran; hexane at 0℃; Inert atmosphere; | 69% |
-
-
623-73-4
diazoacetic acid ethyl ester

-
-
1520-21-4
4-vinyl benzylamine

-
-
15116-32-2
4-(4-amino-phenyl)-butyric acid ethyl ester

| Conditions | Yield |
|---|---|
| With p-cresol; tris(bipyridine)ruthenium(II) dichloride hexahydrate In dichloromethane at 25℃; for 12h; Irradiation; | 97% |
| With tris(bipyridine)ruthenium(II) dichloride hexahydrate; diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate; para-thiocresol In dichloromethane at 25℃; for 12h; Inert atmosphere; Irradiation; | 97% |

| Conditions | Yield |
|---|---|
| With platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex In dichloromethane at 0 - 30℃; for 14h; | 96.6% |
-
-
677-67-8
fluorosulfonyldifluoroacetyl fluoride

-
-
1520-21-4
4-vinyl benzylamine

-
-
1402074-25-2
1,1-difluoro-2-oxo-2-(4-vinylphenylamino)-1-ethanesulfonyl fluoride

| Conditions | Yield |
|---|---|
| With pyridine; hydrogenchloride In dichloromethane | 96% |
| With pyridine In dichloromethane at 0 - 20℃; for 4h; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-vinyl benzylamine With N-ethyl-N,N-diisopropylamine In acetonitrile for 0.0833333h; Inert atmosphere; Stage #2: bromoacetic acid methyl ester In acetonitrile at 55℃; for 48h; Inert atmosphere; | 96% |
-
-
1520-21-4
4-vinyl benzylamine

-
-
18036-87-8
(cyclohexyl)phenylsilane

| Conditions | Yield |
|---|---|
| With platinum(IV) oxide In 1,2-dichloro-ethane at 0 - 20℃; for 16h; | 95.8% |
-
-
1520-21-4
4-vinyl benzylamine

| Conditions | Yield |
|---|---|
| Stage #1: 4-vinyl benzylamine With n-Butyl nitrite In water at 29℃; Acidic conditions; Flow reactor; Stage #2: With lithium thiosulfate In water at 108 - 110℃; Stage #3: With hydrogenchloride In water at 120℃; | 95.4% |

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate In isopropyl alcohol; toluene at 0 - 25℃; for 16h; | 95.3% |

| Conditions | Yield |
|---|---|
| With dihydrogen hexachloroplatinate In isopropyl alcohol; toluene at 5 - 20℃; for 12h; | 95.3% |

| Conditions | Yield |
|---|---|
| With bis(benzonitrile)palladium(II) dichloride In benzene at 5 - 30℃; for 14h; | 95.1% |
-
-
1520-21-4
4-vinyl benzylamine

-
-
501-53-1
benzyl chloroformate

-
-
227778-64-5
N-benzyloxycarbonyl-4-aminostyrene

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine In dichloromethane at 0 - 20℃; Substitution; | 95% |

| Conditions | Yield |
|---|---|
| With diethyl aluminiumcholoride; (3aR,8aR)-6-(2-(tert-butyl)-6-(diphenylphosphanyl)-phenoxy)-2,2-dimethyl-4,4,8,8-tetraphenyltetrahydro-[1,3]dioxol[4,5-e][1,3,2]dioxaphosphepin-cobaltdichloride In hexane; dichloromethane at -20℃; under 900.09 Torr; for 8h; Inert atmosphere; Schlenk technique; enantioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| In methanol; isopropyl alcohol at 0℃; for 1.5h; | 95% |
-
-
1520-21-4
4-vinyl benzylamine

| Conditions | Yield |
|---|---|
| With bis(benzonitrile)palladium(II) dichloride In 1,2-dichloro-ethane at 0 - 25℃; for 16h; | 94.3% |
-
-
591-50-4
iodobenzene

-
-
1520-21-4
4-vinyl benzylamine

-
-
406729-10-0
N,N-diphenyl-4-(2-phenylethenyl)benzenamine

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; 2,8,9-tris(2-methylpropyl)-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane; tris(dibenzylideneacetone)dipalladium (0) In toluene at 100℃; for 24h; | 94% |
| With sodium t-butanolate; tris-(dibenzylideneacetone)dipalladium(0); 2,8,9-tris(2-methylpropyl)-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane In toluene at 100 - 110℃; for 16 - 24h; Product distribution / selectivity; Buchwald-Hartwig amination/intermolecular Heck reaction; | 93% |
-
-
1520-21-4
4-vinyl benzylamine

-
-
28920-43-6
(fluorenylmethoxy)carbonyl chloride

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In 1,4-dioxane at 0 - 23℃; | 94% |
-
-
1520-21-4
4-vinyl benzylamine

-
-
17873-10-8
1-butyl(phenyl)silane

| Conditions | Yield |
|---|---|
| With bis(benzonitrile)palladium(II) dichloride In toluene at 5 - 35℃; for 12h; | 93.8% |
-
-
1520-21-4
4-vinyl benzylamine

-
-
144498-71-5
(3E)-3-[(1,3-dimethyl-1H-indol-2-yl)methylene]dihydro-4-(1-methylethylidene)-2,5-furandione

-
-
253325-55-2
2-[1,3-dimethylindol-2-ylmethylidene]-3-isopropylidene-N-(4-vinylphenyl)succinimide

| Conditions | Yield |
|---|---|
| With 1,1,1,3,3,3-hexamethyl-disilazane; zinc(II) chloride In benzene Heating; | 93% |

| Conditions | Yield |
|---|---|
| (acac)Rh(DPPE) In benzene-d6 | A 2 % Spectr. B 93% |
| RhCl(PPh3)3 In benzene-d6 | A 98 % Spectr. B 2 % Spectr. |

-
-
1520-21-4
4-vinyl benzylamine

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

| Conditions | Yield |
|---|---|
| With bis(cyclopentadienyl)dihydrozirconium In toluene at 25℃; Inert atmosphere; Glovebox; | 93% |
4-Aminostyrene Specification
The CAS registry number of 4-Aminostyrene is 1520-21-4. Its EINECS registry number is 216-185-8. The IUPAC name is 4-ethenylaniline. In addition, the molecular formula is C8H9N and the molecular weight is 119.16. It is also called Benzenamine,4-ethenyl-. What's more, it is a kind of liquid and belongs to the classes of Styrenes; Fluorenes, etc. (reagent for high-performance polymer research); Reagent for High-Performance Polymer Research; Functional Materials.
Physical properties about this chemical are: (1)ACD/LogP: 1.42; (2)ACD/LogD (pH 5.5): 1.35; (3)ACD/LogD (pH 7.4): 1.42; (4)ACD/BCF (pH 5.5): 6.02; (5)ACD/BCF (pH 7.4): 7.02; (6)ACD/KOC (pH 5.5): 120.32; (7)ACD/KOC (pH 7.4): 140.37; (8)#H bond acceptors: 1; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 2; (11)Polar Surface Area: 3.24 Å2; (12)Index of Refraction: 1.621; (13)Molar Refractivity: 41.41 cm3; (14)Molar Volume: 117.6 cm3; (15)Polarizability: 16.41 ×10-24cm3; (16)Surface Tension: 41.1 dyne/cm; (17)Density: 1.012 g/cm3; (18)Flash Point: 108.6 °C; (19)Enthalpy of Vaporization: 47.57 kJ/mol; (20)Boiling Point: 238.8 °C at 760 mmHg; (21)Vapour Pressure: 0.0415 mmHg at 25°C.
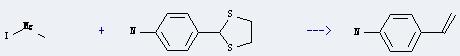
Preparation of 4-Aminostyrene: it can be prepared by methylmagnesium iodide and 2-(4-aminophenyl)-1,3-dithiolane. This reaction will need reagent NiCl2(dppe) and solvents tetrahydrofuran and diethyl ether. The reaction time is 16 hours by heating. The yield is about 60%.
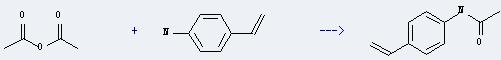
Uses of 4-Aminostyrene: it can react with acetic acid anhydride to get acetic acid-(4-vinyl-anilide). This reaction will need reagent pyridine and solvent CH2Cl2. The yield is about 85%. This reaction is a kind of acetylation reaction.
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes, respiratory system and skin. It may cause sensitization by skin contact. And it has danger of serious damage to health by prolonged exposure through inhalation, and in contact with skin and if swallowed. It has danger of cumulative effects. There is limited evidence of a carcinogenic effect. When you are using it, wear suitable protective clothing and gloves. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: C=C\c1ccc(N)cc1
(2)InChI: InChI=1/C8H9N/c1-2-7-3-5-8(9)6-4-7/h2-6H,1,9H2
(3)InChIKey: LBSXSAXOLABXMF-UHFFFAOYAN
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View