-
Name
4-Bromo-1-butene
- EINECS 225-937-4
- CAS No. 5162-44-7
- Article Data44
- CAS DataBase
- Density 1.312 g/cm3
- Solubility Miscible with water, benzene, ether and ethanol.
- Melting Point -115.07°C (estimate)
- Formula C4H7Br
- Boiling Point 99.3 °C at 760 mmHg
- Molecular Weight 135.004
- Flash Point 9.4 °C
- Transport Information UN 1993 3/PG 2
- Appearance colourless liquid
- Safety 26-36/37/39
- Risk Codes 11-36/37-42/43
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xn,
Xn,  Xi
Xi
- Synonyms 1-Bromo-3-butene;3-Butenyl bromide;4-Bromobutene-1;Allylcarbinyl bromide;Homoallyl bromide;
- PSA 0.00000
- LogP 1.95740
Synthetic route

| Conditions | Yield |
|---|---|
| With lithium bromide In acetone for 1h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide at 200 - 210℃; | 97% |
| With N,N,N,N,N,N-hexamethylphosphoric triamide at 210℃; | 95% |
| With N,N,N,N,N,N-hexamethylphosphoric triamide for 1h; Heating; | 82% |
-
-
38771-21-0
4-bromobut-1-yne

-
-
5162-44-7
1-bromo-4-butene

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In hexane at 40℃; | 90% |
-
-
13287-42-8
(2-bromoethyl)oxirane

-
-
6773-29-1
dimethyl diazomalonate

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
3298-40-6
mesoxalate de methyle

| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate In toluene for 0.75h; Heating; | A 84% B n/a |


| Conditions | Yield |
|---|---|
| With phosphorus tribromide In pyridine | 80% |
| With pyridine; phosphorus tribromide In neat (no solvent) at 0 - 20℃; for 2h; Inert atmosphere; Schlenk technique; | 74% |
| With pyridine; phosphorus tribromide at 0℃; |

| Conditions | Yield |
|---|---|
| With phosphorus tribromide | 73% |

| Conditions | Yield |
|---|---|
| With C50H44Au2P4(2+)*2C2F6NO4S2(1-) In water; acetonitrile for 0.166667h; Inert atmosphere; Sealed tube; UV-irradiation; | 72% |

| Conditions | Yield |
|---|---|
| With NbCl3(N,N′-bis-(2,6-diisopropylphenyl)-1,4-diaza-2,3-dimethyl-1,3-butadiene) In benzene-d6 at 120℃; for 14h; Catalytic behavior; Inert atmosphere; Schlenk technique; Glovebox; | A 59% B 33% |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
106-95-6
allyl bromide

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
7051-34-5
cyclopropylcarbinyl bromide

| Conditions | Yield |
|---|---|
| With copper(I) chloride * phenylphosphite at -20 - -10℃; | A 58% B 1.2% |


| Conditions | Yield |
|---|---|
| With hydrogen bromide at 0℃; | |
| With phosphorus tribromide | |
| With hydrogen bromide |
-
-
106-99-0
buta-1,3-diene

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4784-77-4, 29576-14-5, 39616-19-8
(E/Z)-crotyl bromide

| Conditions | Yield |
|---|---|
| With sulfuric acid; hydrogen bromide |
-
-
74-85-1
ethene

-
-
75-09-2
dichloromethane

-
-
594-11-6
methylcyclopropane

-
-
82621-80-5
2,2-dimethyl-N-bromoglutarimide

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
1194-33-8
2,2-dimethylglutarimide

-
C
-
75-27-4
bromodichloromethane

-
E
-
106-93-4
ethylene dibromide

-
F
-
7051-34-5
cyclopropylcarbinyl bromide

| Conditions | Yield |
|---|---|
| at 15℃; for 1h; Product distribution; Kinetics; Mechanism; Irradiation; | A 1.7 % Chromat. B 50.4 % Chromat. C 12.8 % Chromat. D 50.1 % Chromat. E 2.0 % Chromat. F 18.0 % Chromat. |

-
-
75-09-2
dichloromethane

-
-
594-11-6
methylcyclopropane

-
-
82621-80-5
2,2-dimethyl-N-bromoglutarimide

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
1194-33-8
2,2-dimethylglutarimide

-
C
-
75-27-4
bromodichloromethane

-
D
-
107-80-2
1,3-dibromobutane

-
E
-
7051-34-5
cyclopropylcarbinyl bromide

| Conditions | Yield |
|---|---|
| at 15℃; for 1h; Product distribution; Mechanism; Kinetics; Irradiation; | A 3.2 % Chromat. B 95.9 % Chromat. C 24.1 % Chromat. D 8.5 % Chromat. E 34.5 % Chromat. |

-
-
75-62-7
Bromotrichloromethane

-
-
104877-17-0
(cyclopropylmethyl)(1-hydroxy-1-methylethyl)diazene

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
117153-45-4
trans-1-bromo-5,5,5-trichloro-2-pentene

-
C
-
117135-60-1
3,5-dibromo-1,1,1-trichloropentane

-
D
-
7051-34-5
cyclopropylcarbinyl bromide

-
E
-
67-64-1
acetone

-
F
-
627-70-3
propan-2-one azine

| Conditions | Yield |
|---|---|
| In dichloromethane at -20.1 - 0.4℃; Product distribution; Rate constant; Irradiation; investigation of thermolysis and photolysis; various solvents, time, temperatures, and concentrations; |


| Conditions | Yield |
|---|---|
| nickel at 16.9℃; under 0.08 Torr; Product distribution; |
-
-
157-33-5
bicyclo[1.1.0]butane

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4399-47-7
Bromocyclobutane

-
C
-
7051-34-5
cyclopropylcarbinyl bromide

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate; mercury dibromide 1.) CH2Cl2; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; | |
| With hydrogen bromide Product distribution; influence of reaction time; |


-
-
627-27-0
homoalylic alcohol

-
-
7664-93-9
sulfuric acid

-
-
10035-10-6, 12258-64-9
hydrogen bromide

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4784-77-4, 29576-14-5, 39616-19-8
(E/Z)-crotyl bromide

-
-
627-27-0
homoalylic alcohol

-
-
10035-10-6, 12258-64-9
hydrogen bromide

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
107-80-2
1,3-dibromobutane

| Conditions | Yield |
|---|---|
| at 0℃; |

-
-
627-27-0
homoalylic alcohol

-
-
7789-60-8
phosphorus tribromide

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
107-80-2
1,3-dibromobutane

-
-
2516-33-8
Cyclopropylmethanol

-
-
10035-10-6, 12258-64-9
hydrogen bromide

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4399-47-7
Bromocyclobutane

-
C
-
7051-34-5
cyclopropylcarbinyl bromide

-
-
75-09-2
dichloromethane

-
-
2516-33-8
Cyclopropylmethanol

-
-
7789-60-8
phosphorus tribromide

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4399-47-7
Bromocyclobutane

-
C
-
7051-34-5
cyclopropylcarbinyl bromide

-
-
5911-08-0
chloro(cyclopropyl)methane

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4399-47-7
Bromocyclobutane

-
C
-
7051-34-5
cyclopropylcarbinyl bromide

| Conditions | Yield |
|---|---|
| With sodium bromide; NaY zeolite In pentane at 25℃; Kinetics; | A 58 % Chromat. B 32 % Chromat. C 10 % Chromat. |


| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 20℃; for 3h; |
-
-
2516-33-8
Cyclopropylmethanol

-
A
-
5162-44-7
1-bromo-4-butene

-
B
-
4399-47-7
Bromocyclobutane

-
C
-
7051-34-5
cyclopropylcarbinyl bromide

| Conditions | Yield |
|---|---|
| With hydrogen bromide; 3-ethyl-1-methyl-1H-imidazol-3-ium bromide at 80℃; for 0.333333h; Reagent/catalyst; Temperature; |

-
-
5162-44-7
1-bromo-4-butene

-
-
113738-20-8
4-azido-1-butene

| Conditions | Yield |
|---|---|
| With sodium azide In tetrahydrofuran; water at 80℃; for 3h; Inert atmosphere; | 100% |
| With sodium azide In dimethyl sulfoxide for 18h; Ambient temperature; | 76% |
| With sodium azide In water; acetone for 20h; Heating; |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In acetone Reflux; | 100% |
| With potassium carbonate In acetone Reflux; | 96% |
| 91% |
-
-
5162-44-7
1-bromo-4-butene

-
-
603-35-0
triphenylphosphine

-
-
16958-42-2
but-3-en-1-yltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| In toluene for 24h; Inert atmosphere; Reflux; | 100% |
| In N,N-dimethyl-formamide for 3h; Heating; | 84% |
| In acetonitrile at 23℃; for 23h; Reflux; Inert atmosphere; | 81% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-4-butene With magnesium In tetrahydrofuran at 0 - 20℃; Inert atmosphere; Stage #2: formic acid ethyl ester In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 100% |
| Stage #1: 1-bromo-4-butene With iodine; magnesium In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Stage #2: formic acid ethyl ester In tetrahydrofuran at 0 - 20℃; for 3h; Inert atmosphere; | 98% |
| Stage #1: 1-bromo-4-butene With iodine; magnesium In tetrahydrofuran at 20℃; Inert atmosphere; Stage #2: formic acid ethyl ester In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 94% |
-
-
5162-44-7
1-bromo-4-butene

-
-
2350-46-1
2',3'-dichloro-4'-hydroxybutyrophenone

-
-
113239-47-7
2,3-dichloro-4-(3-butenyloxy)butyrophenone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60 - 65℃; for 5h; | 100% |
-
-
5162-44-7
1-bromo-4-butene

-
-
1121-89-7
piperidine-2,6-dione

-
-
67310-69-4
1-But-3-enylpiperidine-2,6-dione

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In benzene for 12h; Heating; | 100% |
| With sodium hydride In tetrahydrofuran; N,N-dimethyl-formamide |
-
-
5162-44-7
1-bromo-4-butene

-
-
38443-93-5
bis(3-butenyl)magnesium

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-4-butene With magnesium In tetrahydrofuran at 27℃; for 1h; Inert atmosphere; Schlenk technique; Glovebox; Stage #2: In tetrahydrofuran; 1,4-dioxane at 27℃; Inert atmosphere; Schlenk technique; Glovebox; | 100% |
| With 1,4-dioxane; magnesium In diethyl ether for 0.666667h; Metallation; | |
| Stage #1: 1-bromo-4-butene With magnesium In diethyl ether for 0.666667h; Stage #2: With 1,4-dioxane In diethyl ether for 0.5h; |
-
-
5162-44-7
1-bromo-4-butene

| Conditions | Yield |
|---|---|
| With sodium sulfite In water at 90℃; for 16h; Inert atmosphere; | 100% |
| With sodium sulfite In water for 12h; Heating; | |
| With sodium sulfite In water at 70℃; |
-
-
5162-44-7
1-bromo-4-butene

-
-
141-43-5
ethanolamine

-
-
785034-63-1
N-[2'-(hydroxy)ethyl]-1-amino-but-3-ene

| Conditions | Yield |
|---|---|
| With sodium iodide In methanol for 2h; Inert atmosphere; Reflux; | 100% |
| With sodium iodide In methanol for 3h; Reflux; | 40% |
| With sodium iodide In methanol for 3h; Reflux; | |
| With sodium iodide In methanol for 3h; Reflux; |
-
-
5162-44-7
1-bromo-4-butene

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-4-butene With magnesium In tetrahydrofuran at 70℃; for 1.5h; Stage #2: 8-methylene-4-(prop-1-ynyl)octahydro-1H-quinolizine-4-carbonitrile In tetrahydrofuran; dichloromethane at -78 - 20℃; | 100% |
-
-
616-47-7
1-methyl-1H-imidazole

-
-
5162-44-7
1-bromo-4-butene

-
-
943611-02-7
3-(but-3-en-1-yl)-1-methyl-1H-imidazol-3-ium bromide

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; | 100% |
| at 40℃; for 16h; Inert atmosphere; neat (no solvent); | 97% |
| at 40℃; | |
| In toluene at 110℃; | |
| In neat (no solvent) at 100℃; for 0.333333h; Microwave irradiation; Sealed tube; |
-
-
5162-44-7
1-bromo-4-butene

-
-
142851-03-4
1-tert-butyl 4-ethyl piperidine-1,4-dicarboxylate

-
-
146603-99-8
1-(1,1-dimethylethyl) 4-ethyl 4-(2-propenyl)-1,4-piperidinedicarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 1-tert-butyl 4-ethyl piperidine-1,4-dicarboxylate With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 0.75h; Stage #2: 1-bromo-4-butene With N,N,N,N,N,N-hexamethylphosphoric triamide In tetrahydrofuran; hexane at -78 - 20℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex In 5,5-dimethyl-1,3-cyclohexadiene; toluene at 80℃; | 100% |
-
-
5162-44-7
1-bromo-4-butene

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; for 48h; | 100% |
-
-
5162-44-7
1-bromo-4-butene

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; for 72h; | 100% |
-
-
5162-44-7
1-bromo-4-butene

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 16h; Inert atmosphere; Sealed tube; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 16h; Inert atmosphere; Sealed tube; | 64% |
-
-
5162-44-7
1-bromo-4-butene

-
-
112275-50-0
tert-butyl 1,4-diazepine-1-carboxylate

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In tetrahydrofuran Reflux; | 100% |
-
-
5162-44-7
1-bromo-4-butene

| Conditions | Yield |
|---|---|
| Stage #1: 1-bromo-4-butene With magnesium In tetrahydrofuran Reflux; Stage #2: N-tert-butoxycarbonyl-(R)-3-(benzyloxy)-1-[methoxy(methyl)amino]-2-amino-1-oxopropan In tetrahydrofuran at -20 - 20℃; for 18h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 4,4'-dimercaptodiphenyl sulfide With sodium hydroxide In water; N,N-dimethyl-formamide Inert atmosphere; Cooling with ice; Stage #2: 1-bromo-4-butene In water; N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetone at 85℃; for 72h; Inert atmosphere; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide for 24h; Inert atmosphere; | 20% |
-
-
5162-44-7
1-bromo-4-butene

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In butanone for 24h; Reflux; | 100% |
-
-
5162-44-7
1-bromo-4-butene

-
-
144978-35-8, 144978-12-1
(S)-ethyl N-tert-butoxycarbonylpyroglutamate

| Conditions | Yield |
|---|---|
| With magnesium In tetrahydrofuran at -78℃; Grignard reaction; Inert atmosphere; | 99.7% |
| Stage #1: 1-bromo-4-butene; (S)-ethyl N-tert-butoxycarbonylpyroglutamate With magnesium In tetrahydrofuran Stage #2: In tetrahydrofuran at -60℃; | 75% |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In toluene for 16h; Heating; | 99.5% |
| Stage #1: Succinimide With sodium hydride In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 1-bromo-4-butene In N,N-dimethyl-formamide at 50℃; for 20h; | 92% |
| With sodium ethanolate In ethanol for 6h; Heating; | |
| With sodium hydride In tetrahydrofuran; N,N-dimethyl-formamide | |
| Stage #1: Succinimide With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 1h; Inert atmosphere; Stage #2: 1-bromo-4-butene In N,N-dimethyl-formamide at 50℃; for 16h; Inert atmosphere; | 130 g |
-
-
5162-44-7
1-bromo-4-butene

-
-
133733-15-0
1-potassio-2-nitroimidazole salt

-
-
93272-52-7
1-(3-buten-1-yl)-2-nitroimidazole

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether In acetonitrile for 384h; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 12h; Reflux; | 99% |
| Stage #1: 4-cyanophenol With potassium carbonate for 1h; Stage #2: 1-bromo-4-butene at 60℃; | 58% |
| With potassium carbonate In acetonitrile Heating; | 43% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane for 12h; Inert atmosphere; Reflux; | 99% |
| With 18-crown-6 ether; potassium carbonate; potassium iodide In acetonitrile at 75℃; for 18h; Inert atmosphere; | 97% |
| With potassium carbonate; sodium iodide In acetonitrile Reflux; | 41% |
-
-
5162-44-7
1-bromo-4-butene

-
-
180982-83-6
(3S,5aR,8aS,8bR)-3-Isopropyl-8b-methyl-octahydro-cyclopenta[3,4]pyrrolo[2,1-b]oxazol-5-one

-
-
180871-75-4
(3S,5aS,8aS,8bR)-5a-But-3-enyl-3-isopropyl-8b-methyl-octahydro-cyclopenta[3,4]pyrrolo[2,1-b]oxazol-5-one

| Conditions | Yield |
|---|---|
| With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; lithium diisopropyl amide In tetrahydrofuran 1.) -78 deg C up to 0 deg C; 2.) 0 deg C; 3.) -78 deg C; 4.) 0 deg C; | 99% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 12h; Reflux; | 99% |
| With potassium carbonate In acetone for 12h; Inert atmosphere; Reflux; | 95% |
| With potassium carbonate In acetonitrile for 12h; Reflux; | 85% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 18h; Heating; | 99% |
| With potassium carbonate In acetonitrile for 12h; Reflux; | 99% |
| With potassium carbonate In acetonitrile | 97% |
-
-
5162-44-7
1-bromo-4-butene

-
-
188400-93-3
1-[2-(3-fluorophenyl)ethyl]piperazine

-
-
862158-39-2
1-but-3-enyl-4-[2-(3-fluoro-phenyl)-ethyl]piperazine

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 12h; Heating; | 99% |
4-Bromo-1-butene Chemical Properties
Product Name: 4-Bromo-1-butene (CAS NO.5162-44-7)
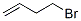
Molecular formula: C4H7Br
Molecular Weight: 135
EINECS: 225-937-4
Boiling point: 98-100 °C(lit.)
Flash point: 49 °F
Density: 1.33 g/mL at 25 °C(lit.)
Refractive index: n20/D 1.462(lit.)
Storage temp.: 2-8°C
Sensitive: Light Sensitive
Index of Refraction: 1.457
Molar Refractivity: 28.03 cm3
Molar Volume: 102.8 cm3
XLogP3-AA: 2.1
H-Bond Donor: 0
H-Bond Acceptor: 0
Structure Descriptors of 4-Bromo-1-butene (CAS NO.5162-44-7):
IUPAC Name: 4-bromobut-1-ene
Canonical SMILES: C=CCCBr
InChI: InChI=1S/C4H7Br/c1-2-3-4-5/h2H,1,3-4H2
InChIKey: DMAYBPBPEUFIHJ-UHFFFAOYSA-N
Product Categories: Halides; omega-Functional Alkanols, Carboxylic Acids, Amines & Halides; omega-Unsaturated Halides; Alkenyl; Halogenated Hydrocarbons; Organic Building Blocks
4-Bromo-1-butene Uses
4-Bromo-1-butene (CAS NO.5162-44-7) is used for drug intermediates.
4-Bromo-1-butene Consensus Reports
4-Bromo-1-butene (CAS NO.5162-44-7) is reported in EPA TSCA Inventory.
4-Bromo-1-butene Safety Profile
Safety Information of 4-Bromo-1-butene (CAS NO.5162-44-7):
Hazard Codes: F , Xn
, Xn ,Xi
,Xi Xi
Xi
Risk Statements information:
11: Highly Flammable
36/37: Irritating to eyes and respiratory system
42/43: May cause sensitization by inhalation and skin contact
Safety Statements information:
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
F: 8-19
HazardClass: 3
PackingGroup: II
HS Code: 29033036
RIDADR: UN 1993 3/PG 2
WGK Germany: 3
A dangerous fire hazard (flash point <1°C). Violent reaction with chloromethylphenylsilane + chloroplatinic acid. When heated to decomposition it emits toxic fumes of Br−.
4-Bromo-1-butene Specification
4-Bromo-1-butene , its CAS NO. is 5162-44-7, the synonyms are 4-bromobut-1-ene ; 4-Bromobutene ; 4-Bromobutene-(1) ; Homoallyl bromide ; 4-bromo-1-buten ; 4-bromo-but-1-ene ; 1-Bromo-3-butene;1-butene,4-bromo- .
Related Products
- 4-Bromo-1-butene
- 51627-14-6
- 51627-47-5
- 51628-12-7
- 5162-82-3
- 5162-90-3
- 51630-33-2
- 51630-58-1
- 51631-50-6
- 5163-20-2
- 51632-16-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View