-
Name
4-Bromobenzyl alcohol
- EINECS 212-851-7
- CAS No. 873-75-6
- Article Data282
- CAS DataBase
- Density 1.565 g/cm3
- Solubility 2.31g/L at 20℃
- Melting Point 75-77 °C
- Formula C7H7BrO
- Boiling Point 267.8 °C at 760 mmHg
- Molecular Weight 187.036
- Flash Point 115.7 °C
- Transport Information
- Appearance White to slightly beige crystal
- Safety 24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Benzylalcohol, p-bromo- (6CI,7CI,8CI);(4-Bromophenyl)methanol;(p-Bromophenyl)methanol;1-(p-Bromophenyl)methanol;4-Bromobenzenemethanol;p-Bromobenzyl alcohol;4-Hydroxymethyl-1-bromobenzene;
- PSA 20.23000
- LogP 1.94140
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogen; Et4N In 1,2-dimethoxyethane at 100℃; under 38000 Torr; for 13h; | 100% |
| With tri-n-butyl-tin hydride In methanol; diethyl ether for 4h; Reduction; Heating; | 100% |
| With hydrogen; phosphotungstic acid 44-hydrate; [Fe(C5H4P(i-Pr)2)2Rh(C8H12)]BF4 In water; isopropyl alcohol at 20℃; under 5171.62 Torr; for 16h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium bis(2-methoxyethoxy)aluminium dihydride In toluene 1.) 10-20 deg C, 2.) room temperature, 10 min; | 100% |
| With diisobutylaluminium hydride In toluene at 0℃; for 0.5h; Inert atmosphere; | 98% |
| Stage #1: 4-methoxycarbonylphenyl bromide With diethylzinc; lithium chloride In tetrahydrofuran; hexane at 20℃; for 6h; Inert atmosphere; Stage #2: With sodium hydroxide In tetrahydrofuran; hexane; water at 20℃; for 8h; Inert atmosphere; chemoselective reaction; | 98% |

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With water | 100% |
-
-
17100-68-4
2-((4-bromobenzyl)oxy)tetrahydro-2H-pyran

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| silica-supported prop-1-ylsulfonic acid In methanol | 99.8% |
| With sulfuric acid; silica gel In methanol at 20℃; for 0.5h; | 98% |
| With trichloroisocyanuric acid In methanol at 20℃; for 4h; | 95% |

| Conditions | Yield |
|---|---|
| With C30H34Cl2N2P2Ru; potassium methanolate; hydrogen In tetrahydrofuran at 100℃; under 38002.6 - 76005.1 Torr; for 15h; Glovebox; Autoclave; | 98% |
| With LiPh2InH2 In diethyl ether for 24h; Ambient temperature; | 95% |
| Stage #1: Ethyl 4-bromobenzoate With trimethylsilyl trifluoromethanesulfonate; triphenylphosphine; 1-Phenylbut-1-en-3-one In dichloromethane at -78 - 0℃; for 1h; Inert atmosphere; Stage #2: With diisobutylaluminium hydride In hexane; dichloromethane at -78℃; Inert atmosphere; Stage #3: With potassium carbonate In methanol; hexane; dichloromethane at 20℃; for 0.5h; Inert atmosphere; | 93% |
-
-
86605-93-8
(4-bromobenzyloxy)trimethylsilane

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With methanol; 1,3-disulfonic acid imidazolium hydrogen sulfate at 20℃; for 0.0833333h; Green chemistry; | 98% |
| With rice husk ash supported on anatase-phase titania nanoparticles nanocomposite In methanol at 20℃; for 0.05h; | 97% |
| With poly (ethylene glycol)-sulfonated sodium montmorillonite nanocomposite In methanol at 20℃; for 0.0666667h; | 96% |
-
-
21388-92-1
4-bromobenzyl acetate

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With magnesium methanolate In methanol Ambient temperature; | 96% |
| With water at 20℃; for 0.416667h; | 96% |
| With potassium hydroxide |
-
-
130534-91-7
1-bromo-4-[(methoxymethyloxy)methyl]benzene

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With 1-methylimidazole hydrogen sulfate at 120℃; for 0.0166667h; Microwave irradiation; chemoselective reaction; | 96% |
| With bismuth(III) chloride; water In acetonitrile at 50℃; for 10h; | 88% |
| With niobium pentachloride In acetonitrile at 0 - 20℃; for 1h; | 81% |
| phosphotungstic acid In ethanol for 6h; Heating; | 73% |
-
-
216443-67-3
4-bromo-1-[(ethoxymethoxy)methyl]benzene

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With 1-methylimidazole hydrogen sulfate at 120℃; for 0.0166667h; Microwave irradiation; chemoselective reaction; | 95% |
| phosphotungstic acid In ethanol for 5.5h; Heating; | 82% |
-
-
1140924-37-3
(E)-6-(4-bromobenzyloxy)hex-3-en-2-one

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 22℃; for 1h; | 94% |
-
-
87736-74-1
4-(tert-butyldimethylsiloxymethyl)bromobenzene

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With potassium hydrogen difluoride In methanol at 60℃; for 16h; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-Bromobenzoic acid With 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane at 20℃; for 12h; Inert atmosphere; Stage #2: With silica gel In methanol at 50℃; for 3h; | 93% |
| Stage #1: 4-Bromobenzoic acid With sodium aminodiboranate In tetrahydrofuran at 20℃; Stage #2: With water | 90% |
| With potassium borohydride; hafnium tetrachloride In tetrahydrofuran at 40℃; for 18.6h; Inert atmosphere; Cooling with ice; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromo-benzaldehyde With potassium hydroxide for 0.0833333h; Cannizzaro Reaction; Milling; Inert atmosphere; Sealed tube; Green chemistry; Stage #2: With hydrogenchloride In water Green chemistry; | A 92% B 92% |
| With sodium hydroxide In water at 15℃; for 3h; Cannizzaro Reaction; | A 90% B n/a |
| With [(hydrotris(3,5-diphenylpyrazole-1-yl)borate)-FeII(benzilate)]; oxygen In benzene at 20℃; Inert atmosphere; | A 85% B 40% |

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Schlenk technique; Inert atmosphere; | 92% |
| With sodium hydroxide In water |

| Conditions | Yield |
|---|---|
| With potassiumhexacyanoferrate(II) trihydrate; C30H27FeN2OP; water; palladium diacetate; sodium carbonate In 1,4-dioxane at 100℃; for 3h; Inert atmosphere; Schlenk technique; | 90% |
| With oxygen; eosin y In dimethyl sulfoxide at 25℃; for 12h; Irradiation; | 81% |
| With lead(II) oxide; acetic acid Behandeln des Reaktionsprodukts mit methanol. KOH; |
-
-
1122-91-4
4-bromo-benzaldehyde

-
A
-
106-38-7
para-bromotoluene

-
B
-
873-75-6
4-bromobenzenemethanol

-
C
-
100-52-7
benzaldehyde

-
D
-
100-51-6
benzyl alcohol

| Conditions | Yield |
|---|---|
| With hydrogen; platinum(IV) oxide In water; isopropyl alcohol at 20℃; under 724.026 Torr; for 16h; | A 4% B 90% C 1% D 4% |

-
-
1566593-61-0
2-((4-bromobenzyl)oxy)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With water; silica gel In methanol at 60℃; Inert atmosphere; | 90% |
| With hydrogenchloride In tetrahydrofuran; methanol; water at 50℃; for 1h; Inert atmosphere; Glovebox; Schlenk technique; | 87% |
| With silica gel In methanol at 60℃; for 3h; Inert atmosphere; | 83% |
-
-
19350-68-6
p-bromobenzaldehyde tosylhydrazone

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With water; potassium carbonate at 130℃; for 0.166667h; Microwave irradiation; | 89% |

| Conditions | Yield |
|---|---|
| With formaldehyd; [ruthenium(II)(η6-1-methyl-4-isopropyl-benzene)(chloride)(μ-chloride)]2 In water; toluene at 90℃; | 88% |
| With sulfuric acid; hydrogen; nickel In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| With C27H31ClN3RuSe(1+)*F6P(1-); potassium hydroxide In water at 110℃; for 6h; Reagent/catalyst; Time; | 88% |
-
-
589-15-1
1-bromomethyl-4-bromobenzene

-
-
71-36-3
butan-1-ol

-
A
-
873-75-6
4-bromobenzenemethanol

-
B
-
133842-36-1
1-bromo-4-(butoxymethyl)benzene

| Conditions | Yield |
|---|---|
| With sodium hydroxide; 5,11,17,23-tetramethoxy-25,26,27,28-tetrakis(trimethylammoniomethyl)calix<4>arene tetrachloride at 60℃; for 2h; | A 3% B 87% |

-
-
18469-37-9
4-bromo-N,N-dimethylbenzamide

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With sodium hydride; sodium iodide; zinc(II) iodide In tetrahydrofuran; mineral oil at 40℃; for 7h; Sealed tube; | 87% |
-
-
147441-51-8, 147441-52-9, 77853-37-3
2-(4-bromophenyl)-1,3-oxathiolane

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate; nickel(II) chloride hexahydrate at 20℃; for 2h; | 86% |
-
-
1122-91-4
4-bromo-benzaldehyde

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

-
A
-
873-75-6
4-bromobenzenemethanol

-
B
-
1566593-61-0
2-((4-bromobenzyl)oxy)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With tris(cyclopentadienyl)lanthanum(III) In tetrahydrofuran at 25℃; for 1h; Inert atmosphere; Schlenk technique; Glovebox; | A 86% B n/a |


| Conditions | Yield |
|---|---|
| With C24H20ClN2OPRu; potassium tert-butylate; hydrogen In tetrahydrofuran at 110℃; under 10640.7 Torr; for 36h; Inert atmosphere; Schlenk technique; | 86% |
| With [fac-8-(2-diphenylphosphinoethyl)aminotrihydroquinoline]RuH(η1-BH4)(CO); hydrogen In isopropyl alcohol at 140℃; under 37503.8 Torr; for 24h; Autoclave; | 60 %Chromat. |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In various solvent(s) at 80℃; for 3h; | 84% |
| With aluminum oxide; sodium tetrahydroborate In diethyl ether | |
| With lithium aluminium tetrahydride In diethyl ether |
-
-
55605-27-1
(4-bromophenyl)methylenediacetate

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel(II) chloride hexahydrate In methanol at 20℃; for 2h; chemoselective reaction; | 84% |

| Conditions | Yield |
|---|---|
| With C62H74Cl2N4Pd2S2; caesium carbonate In tetrahydrofuran; water at 50℃; for 2h; Inert atmosphere; Sealed tube; | 83% |
| With di-μ-chloro-bis{2-[3-(2,6-diisopropylphenyl)imidazolin-2-ylidene]-(3-phenylthio)phenyl-κ2C,C’}dipalladium(II); caesium carbonate In tetrahydrofuran; water at 70℃; for 2h; Inert atmosphere; Sealed tube; | 75% |
| With rhodium(III) chloride; triphenylphosphine; sodium hydroxide In tetrahydrofuran at 130℃; for 6h; Autoclave; Inert atmosphere; | 71% |
-
-
1122-91-4
4-bromo-benzaldehyde

-
A
-
873-75-6
4-bromobenzenemethanol

-
B
-
1007224-79-4
2,2-bis(4-bromophenyl)ethanol

| Conditions | Yield |
|---|---|
| With zinc In water at 90 - 160℃; for 2h; Microwave irradiation; Green chemistry; | A 15% B 83% |

| Conditions | Yield |
|---|---|
| With dinitrogen tetraoxide; ferric nitrate for 0.08h; Ambient temperature; further oxidizing agent, further conditions and solvents; | 100% |
| With butyltriphenylphosphonium chlorochromate In acetonitrile for 0.333333h; Heating; | 100% |
| With 1H-imidazole; sodium periodate; manganese(III)-porphyrin complex immobilized on polystyrene In water; acetonitrile at 20℃; for 0.75h; | 100% |
-
-
873-75-6
4-bromobenzenemethanol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
87736-74-1
4-(tert-butyldimethylsiloxymethyl)bromobenzene

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 0 - 20℃; for 1.5h; | 100% |
| With 1H-imidazole In dichloromethane at 20℃; for 12h; | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 72h; | 100% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In tetrahydrofuran at 5 - 30℃; for 15.13h; | 100% |
| With bismuth(lll) trifluoromethanesulfonate In acetonitrile at 20℃; for 0.0833333h; | 99% |
| K5 In acetonitrile at 20℃; for 0.25h; | 99% |
-
-
873-75-6
4-bromobenzenemethanol

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
86605-93-8
(4-bromobenzyloxy)trimethylsilane

| Conditions | Yield |
|---|---|
| With 1-adamanthanol; N,N'-dibromo-N,N'-1,2-ethanediylbis-(benzenesulfonamide) In dichloromethane at 20℃; for 2h; | 100% |
| With tribromomelamine In dichloromethane; acetonitrile at 20℃; for 1.25h; | 98% |
| With 1,3-disulfonic acid imidazolium hydrogen sulfate In neat (no solvent) at 20℃; for 0.0166667h; Green chemistry; | 98% |
-
-
873-75-6
4-bromobenzenemethanol

-
-
4688-76-0
2-Biphenylboronic acid

-
-
6051-49-6
[1,1':2',1"-terphenyl]-4-ylmethanol

| Conditions | Yield |
|---|---|
| With dichlorido[2-(1-methyl-4,5-diphenyl-1H-imidazol-2-yl)pyridine]palladium(II); potassium carbonate In ethanol; water for 0.5h; Suzuki-Miyaura Coupling; Reflux; | 100% |
| With palladium diacetate; potassium carbonate; triphenylphosphine In water; toluene at 110℃; Sealed tube; | 85% |
-
-
116-11-0
2-Methoxypropene

-
-
873-75-6
4-bromobenzenemethanol

-
-
1201913-76-9
4-bromo-1-(1-methoxy-1-methylethoxymethyl)benzene

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In tetrahydrofuran at -20℃; for 15h; Inert atmosphere; | 100% |
-
-
873-75-6
4-bromobenzenemethanol

-
-
98-95-3
nitrobenzene

-
A
-
1122-91-4
4-bromo-benzaldehyde

-
B
-
5877-51-0
N-(4-bromobenzylidene)aniline

| Conditions | Yield |
|---|---|
| With α,α,α-trifluorotoluene; titanium(IV) oxide In dodecane under 750.075 Torr; for 3h; Darkness; Inert atmosphere; Irradiation; | A n/a B 100% |

-
-
873-75-6
4-bromobenzenemethanol

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; triethylamine In tetrahydrofuran at 0 - 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In toluene at 50℃; for 0.666667h; Molecular sieve; | 99.9% |
| With C30H24AgBr4N8(1+)*AgBr2(1-); N-benzyl-trimethylammonium hydroxide In toluene at 20℃; for 12h; Molecular sieve; Schlenk technique; | 98% |
| With oxygen In toluene at 80℃; for 24h; Schlenk technique; | 97% |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
873-75-6
4-bromobenzenemethanol

-
-
17100-68-4
2-((4-bromobenzyl)oxy)tetrahydro-2H-pyran

| Conditions | Yield |
|---|---|
| silica-supported prop-1-ylsulfonic acid In acetonitrile for 0.166667h; | 99.7% |
| Stage #1: 3,4-dihydro-2H-pyran; 4-bromobenzenemethanol With toluene-4-sulfonic acid In dichloromethane at 20℃; for 96h; Stage #2: With potassium carbonate In dichloromethane for 2h; | 98% |
| In dichloromethane at 20℃; for 0.133333h; | 98% |

| Conditions | Yield |
|---|---|
| With bromine; triphenylphosphine In dichloromethane at -78 - 20℃; for 18h; Inert atmosphere; | 99% |
| With 1,1,1,2,2,2-hexamethyldisilane In chloroform for 2h; Reflux; | 98% |
| With phosphorus tribromide In chloroform at 0 - 20℃; for 1h; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromobenzenemethanol With sodium hydride In tetrahydrofuran; paraffin oil at 0 - 20℃; for 1h; Stage #2: methyl iodide In tetrahydrofuran; paraffin oil at 20℃; for 15h; | 99% |
| Stage #1: 4-bromobenzenemethanol With potassium hydroxide In dimethyl sulfoxide at 20℃; for 1h; Stage #2: methyl iodide In dimethyl sulfoxide for 0.166667h; | 99% |
| With potassium hydroxide In dimethyl sulfoxide for 0.5h; Ambient temperature; | 96% |
-
-
13154-24-0
triisopropylsilyl chloride

-
-
873-75-6
4-bromobenzenemethanol

-
-
181717-64-6
4-(triisopropylsilyloxymethyl)bromobenzene

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide for 24h; Ambient temperature; | 99% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 16h; Inert atmosphere; | 99% |
| With 1H-imidazole In N,N-dimethyl-formamide at 21℃; for 1h; | 91% |
| With 1H-imidazole In N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With tribromomelamine In neat (no solvent) at 20℃; for 1h; Time; Solvent; Green chemistry; chemoselective reaction; | 99% |
| With K5 for 0.75h; Heating; | 98% |
| With bismuth(lll) trifluoromethanesulfonate for 0.5h; Heating; | 96% |
-
-
873-75-6
4-bromobenzenemethanol

-
-
545-06-2
trichloroacetonitrile

-
-
146285-52-1
(4-bromobenzyl)-2,2,2-trichloroacetimidate

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromobenzenemethanol With sodium hydride In tetrahydrofuran; mineral oil at 25℃; Inert atmosphere; Stage #2: trichloroacetonitrile In tetrahydrofuran; mineral oil at 0 - 25℃; Inert atmosphere; | 99% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In hexane at 0℃; for 0.5h; | 95% |
| Stage #1: 4-bromobenzenemethanol With sodium hydride In diethyl ether; oil at 20℃; for 0.0833333h; Stage #2: trichloroacetonitrile In diethyl ether; oil at 20℃; for 2h; | 92% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane at 20℃; for 2h; | 84% |
| Stage #1: 4-bromobenzenemethanol In toluene for 0.333333h; Metallation; Stage #2: trichloroacetonitrile at 20℃; for 1h; Addition; | 25% |

| Conditions | Yield |
|---|---|
| Stage #1: carbon disulfide; 4-bromobenzenemethanol With sodium hydride In tetrahydrofuran at 20℃; for 10h; Addition; Stage #2: methyl iodide In tetrahydrofuran at 20℃; for 1h; Methylation; | 99% |
| Stage #1: 4-bromobenzenemethanol With sodium hydroxide In tetrahydrofuran at 20℃; for 0.166667h; Stage #2: carbon disulfide In tetrahydrofuran at 0℃; for 0.166667h; Stage #3: methyl iodide In tetrahydrofuran at 0 - 20℃; | 88% |
| Stage #1: 4-bromobenzenemethanol With 1H-imidazole; sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 0.5h; Inert atmosphere; Stage #2: carbon disulfide In tetrahydrofuran; mineral oil at 20℃; Inert atmosphere; Stage #3: methyl iodide In tetrahydrofuran; mineral oil at 20℃; Inert atmosphere; | 82% |


| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In acetonitrile for 0.5h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With oxygen; potassium carbonate at 60℃; under 750.075 Torr; for 20h; | 99% |
| With potassium carbonate at 60℃; under 750.075 Torr; for 2h; | 94.1% |
| With oxygen at 70℃; under 750.075 Torr; for 16h; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-bromobenzenemethanol With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; tert-butylhypochlorite In dichloromethane at 20℃; for 1h; Inert atmosphere; Stage #2: With ammonia; iodine In dichloromethane; water at 20℃; for 2h; Inert atmosphere; | 99% |
| With ammonium hydroxide; oxygen In tert-Amyl alcohol under 7500.75 Torr; for 16h; Green chemistry; | 99% |
| With ammonia; oxygen In tert-Amyl alcohol; water at 100℃; under 3750.38 Torr; for 4h; Autoclave; High pressure; | 99% |
-
-
873-75-6
4-bromobenzenemethanol

-
-
55883-45-9
4-bromobenzyliodide

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate; hydrogen; iodine; 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl In 1,2-dichloro-ethane at 20℃; under 22502.3 Torr; Autoclave; Schlenk technique; | 99% |
| With bis(1,5-cyclooctadiene)rhodium(I) trifluoromethanesulfonate; Ru(OCOCH3)2{(S)-2,2'-bis(diphenylphosphino)-1,1'-dinaphthyl)}; hydrogen; iodine In toluene at 20℃; under 22502.3 Torr; for 10.5h; | 99% |
| Stage #1: 4-bromobenzenemethanol With 1,2,3-Benzotriazole; thionyl chloride In dichloromethane Stage #2: With potassium iodide In dichloromethane; N,N-dimethyl-formamide for 0.1h; | 96% |

| Conditions | Yield |
|---|---|
| With C20H20N2O2Pd; sodium hydrogencarbonate In water at 80℃; for 6h; Suzuki-Miyaura Coupling; | 99% |
| With trans-di(μ-acetato)bis[o-(di-o-tolylphosphino)benzyl]dipalladium(II); potassium carbonate In water for 0.5h; Catalytic behavior; Suzuki-Miyaura Coupling; Sealed tube; Inert atmosphere; Reflux; | 98% |
| With palladium diacetate; potassium carbonate; urea In water; isopropyl alcohol at 20℃; for 0.333333h; Suzuki-Miyaura Coupling; Green chemistry; | 97% |
-
-
873-75-6
4-bromobenzenemethanol

-
-
124-63-0
methanesulfonyl chloride

-
-
237763-11-0
methanesulfonic acid 4-bromo-benzyl ester

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at -20 - 20℃; for 3h; | 99% |
| With triethylamine In dichloromethane at -50℃; for 0.5h; | 99% |
| Stage #1: 4-bromobenzenemethanol With triethylamine In tetrahydrofuran at 0℃; Stage #2: methanesulfonyl chloride In tetrahydrofuran at 0 - 20℃; | 74% |
-
-
873-75-6
4-bromobenzenemethanol

-
-
1066-54-2
trimethylsilylacetylene

-
-
275386-60-2
4-(hydroxymethyl)-1-(trimethylsilylethynyl)benzene

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; triethylamine at 70℃; for 23h; Inert atmosphere; | 99% |
| With bis-triphenylphosphine-palladium(II) chloride; triethylamine at 70℃; for 36h; Sonogashira Cross-Coupling; | 93% |
| With copper(l) iodide; triethylamine; bis(triphenylphosphine)palladium(II)-chloride at 70℃; for 36h; | 92% |

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; ammonium bicarbonate In water at 100℃; for 24h; Time; Suzuki Coupling; Inert atmosphere; Schlenk technique; | 99% |
| With tetra-butylammonium acetate; Pd EnCat-30TM In ethanol Suzuki cross-coupling; microwave irradiation; | 89% |
-
-
288-32-4
1H-imidazole

-
-
873-75-6
4-bromobenzenemethanol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
87736-74-1
4-(tert-butyldimethylsiloxymethyl)bromobenzene

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide | 99% |
| In dichloromethane |

-
-
288-32-4
1H-imidazole

-
-
873-75-6
4-bromobenzenemethanol

-
-
86718-08-3
1-(Hydroxymethyl)-4-(1H-imidazol-1-yl)benzene

| Conditions | Yield |
|---|---|
| With copper(I) oxide; potassium phosphate; N1,N2-bis(furan-2-ylmethyl)oxalamide In dimethyl sulfoxide at 120℃; for 24h; | 99% |
| With 1-(5,6,7,8-tetrahydroquinolin-8-yl)ethan-1-one; caesium carbonate; copper(I) bromide In dimethyl sulfoxide at 120℃; for 12h; Inert atmosphere; | 80% |
| With copper(l) iodide; 6,7-dihydro-5H-quinolin-8-one oxime; tetrabutylammomium bromide; sodium hydroxide In water at 120℃; for 48h; Inert atmosphere; | 79% |
4-Bromobenzyl alcohol Specification
The Benzenemethanol,4-bromo- with CAS registry number of 873-75-6 is also known as 4-Bromobenzyl alcohol. The IUPAC name is (4-Bromophenyl)methanol. It belongs to product categories of Benzhydrols, Benzyl & Special Alcohols; Alcohol; Alcohols; Bromine Compounds; C7 to C8; Oxygen Compounds. Its EINECS registry number is 212-851-7. In addition, the formula is C7H7BrO and the molecular weight is 187.03. This chemical is a white to slightly beige crystal that may cause inflammation to the skin or other mucous membranes. What's more, it should be sealed in cool, dry place away from oxidants.
Physical properties about Benzenemethanol,4-bromo- are: (1)ACD/LogP: 1.81; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.81; (4)ACD/LogD (pH 7.4): 1.81; (5)ACD/BCF (pH 5.5): 13.89; (6)ACD/BCF (pH 7.4): 13.89; (7)ACD/KOC (pH 5.5): 228.89; (8)ACD/KOC (pH 7.4): 228.89; (9)#H bond acceptors: 1; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Index of Refraction: 1.591; (13)Molar Refractivity: 40.39 cm3; (14)Molar Volume: 119.4 cm3; (15)Surface Tension: 46.1 dyne/cm; (16)Density: 1.565 g/cm3; (17)Flash Point: 115.7 °C; (18)Enthalpy of Vaporization: 53.43 kJ/mol; (19)Boiling Point: 267.8 °C at 760 mmHg; (20)Vapour Pressure: 0.00397 mmHg at 25 °C.
Preparation of Benzenemethanol,4-bromo-: it is prepared by reaction of 4-bromo-benzaldehyde. The reaction needs reagent Zr(BH4)2Cl2(dabco)2 and solvent propan-2-ol with other condition of heating for 2 hours. The yield is about 96 %.

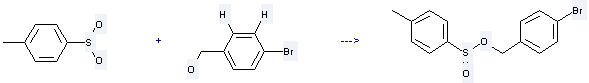
Uses of Benzenemethanol,4-bromo-: it is used to produce 4-methyl-benzenesulfinic acid 4-bromo-benzyl ester by reaction with p-toluenesulfinic acid. The reaction occurs with reagent 1,1'-carbonyldiimidazole and solvent CH2Cl2 for 2 hours. The yield is about 69 %.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC(=CC=C1CO)Br
2. InChI: InChI=1S/C7H7BrO/c8-7-3-1-6(5-9)2-4-7/h1-4,9H,5H2
3. InChIKey: VEDDBHYQWFOITD-UHFFFAOYSA-N
Related Products
- 4-Bromobenzyl acetate
- 4-Bromobenzyl alcohol
- 4-Bromobenzyl bromide
- 4-Bromobenzyl chloride
- 4-Bromobenzyl mercaptan
- 4-Bromobenzyl(2,3-dihydro-1H-indole-7-yl)methanone
- 4-Bromobenzylamine
- 4-Bromobenzylamine hydrochloride
- 4-Bromobenzylboronic acid pinacol ester
- 4-Bromobenzylsulfonyl chloride
- 87376-25-8
- 873-76-7
- 873-77-8
- 873779-30-7
- 873785-69-4
- 87378-88-9
- 873798-09-5
- 873-83-6
- 87385-39-5
- 87387-66-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View