-
Name
4-Fluoro-3-phenoxybenzaldehyde
- EINECS 269-856-2
- CAS No. 68359-57-9
- Article Data6
- CAS DataBase
- Density 1.229 g/cm3
- Solubility
- Melting Point
- Formula C13H9FO2
- Boiling Point 320 °C at 760 mmHg
- Molecular Weight 216.212
- Flash Point 142.5 °C
- Transport Information UN 3082 9/PG 3
- Appearance Light yellowish liquid.
- Safety 61
- Risk Codes 22-51/53
-
Molecular Structure
-
Hazard Symbols
 Xn;
Xn;  N
N
- Synonyms 3-Phenoxy-4-fluorobenzaldehyde;4-Fluoro-3-phenoxybenzaldehyde;
- PSA 26.30000
- LogP 3.43050
Synthetic route
-
-
68359-37-5
cyfluthrin

-
A
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
B
-
55701-05-8
3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol for 2h; Heating; |
-
-
68359-55-7
4-fluoro-3-phenoxybenzyl bromide

-
-
108-03-2
1-Nitropropane

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| In ethanol |
-
-
77771-05-2
4-fluoro-3-phenoxy-benzaldehyde ethyleneacetal

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol; water; toluene |

-
A
-
541-47-9
3-Methylbutenoic acid

-
B
-
60310-82-9
3-(2-chloro-vinyl)-2,2-dimethylcyclopropanecarboxylic acid

-
C
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
E
-
76783-44-3
2-(4-fluoro-3-phenoxyphenyl)-2-hydroxyethanenitrile

-
F
-
77279-89-1
4-fluoro-3-phenoxy benzoic acid

| Conditions | Yield |
|---|---|
| In hexane for 17h; UV-irradiation; |

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
77279-89-1
4-fluoro-3-phenoxy benzoic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid In ethanol for 4h; Heating; | 100% |
| With potassium permanganate In water for 2h; Heating; | 65% |
| With dihydrogen peroxide; acetic acid at 60 - 80℃; for 2h; |
-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
68359-53-5
4-fluoro-3-phenoxyphenylmethanol

| Conditions | Yield |
|---|---|
| With formic acid; C20H29ClIrN4(1+)*Cl(1-) In ethanol; water at 80℃; for 0.25h; chemoselective reaction; | 98% |
| With sodium hydroxide In diethyl ether; water | |
| With sodium hydroxide In diethyl ether; water | |
| With sodium hydroxide In diethyl ether; water |

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In water; benzene at 15 - 20℃; for 10h; | 95% |

-
-
773837-37-9
sodium cyanide

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With triethylamine In water; toluene at 20℃; for 4h; | 95% |


-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With pyridine In cyclohexane; water at 20℃; for 4h; | 93% |

-
-
17176-77-1
Dibenzyl phosphite

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 14h; | 92% |
-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
52314-67-7
permethric acid chloride

-
-
7757-82-6
sodium sulfate

-
-
68359-33-1
3'-phenoxy-4'-fluoro-benzyl 2,2-dimethyl-3-(2,2-dichlorovinyl)-cyclopropane-carboxylate

| Conditions | Yield |
|---|---|
| In water; ethyl acetate | 91.5% |

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
1384471-91-3
2-amino-N'-hydroxychroman-3-carboxamidine

-
-
1384471-90-2
N-(4-fluoro-3-phenoxybenzylidene)-3-(5-(4-fluoro-3-phenoxyphenyl)-1,2,4-oxadiazol-3-yl)-3,4-dihydro-2H-chromen-2-amine

| Conditions | Yield |
|---|---|
| for 0.0833333h; Irradiation; | 91% |
-
-
773837-37-9
sodium cyanide

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With pyridine In cyclohexane; water at 20℃; for 4h; | 91% |

-
-
5112-36-7
pyridine-2,6-dicarbohydrazide

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol Reflux; | 89% |

| Conditions | Yield |
|---|---|
| With ammonium acetate; sodium hydroxide In water at 80℃; for 8h; Green chemistry; | 87% |

-
-
292871-44-4
ethyl 4-[4-(methylthio)phenyl]-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidine-5-carboxylate

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
79-11-8
chloroacetic acid

| Conditions | Yield |
|---|---|
| With sodium acetate; acetic anhydride; acetic acid for 3h; Heating; | 84% |

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
1576-35-8
toluene-4-sulfonic acid hydrazide

| Conditions | Yield |
|---|---|
| In ethanol Reflux; | 84% |

| Conditions | Yield |
|---|---|
| With acetic acid at 50℃; for 5h; Pictet-Spengler Synthesis; Molecular sieve; Inert atmosphere; | 83% |
-
-
27784-76-5
tert-butyl diethylphosphonoacetate

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
1393363-56-8
tert-butyl (E)-3-(3'-phenoxy-4'-fluorophenyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl diethylphosphonoacetate With methylmagnesium bromide In tetrahydrofuran; diethyl ether at 20℃; for 0.25h; Inert atmosphere; Stage #2: 4-fluoro-3-phenoxybenzaldehyde In tetrahydrofuran; diethyl ether for 15h; Horner-Wadsworth-Emmons olefination; Inert atmosphere; Reflux; optical yield given as %de; diastereoselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With sulfated anatase titania In neat (no solvent) at 20℃; for 0.5h; Green chemistry; | 82% |
| With BiCl3-loaded montmorillonite K10 In neat (no solvent) at 20℃; for 0.666667h; Green chemistry; | 82% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
108-59-8
malonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (2-nitroethenyl)benzene; malonic acid dimethyl ester With sodium hydroxide In methanol at 0℃; for 0.75h; Stage #2: 4-fluoro-3-phenoxybenzaldehyde; formamide In methanol Reflux; | 78% |

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 8h; Reflux; | 72% |
-
-
1658-27-1
1,5-dioxaspiro[5.5]undecane-2,4-dione

-
-
102-96-5
(2-nitroethenyl)benzene

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 1,5-dioxaspiro[5.5]undecane-2,4-dione; (2-nitroethenyl)benzene With triethylamine In ethanol at 20℃; for 3h; Michael Addition; Stage #2: 4-fluoro-3-phenoxybenzaldehyde With ammonium acetate In ethanol at 45℃; for 20h; Mannich Aminomethylation; | 71% |


| Conditions | Yield |
|---|---|
| With hydrazine hydrate; morpholine triflate In ethanol; water for 8h; Reflux; Green chemistry; | 67% |

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| In methanol; ethanol at 100℃; for 18h; | 65% |
-
-
3179-10-0
1-methoxy-4-(2-nitro-vinyl)-benzene

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
108-59-8
malonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1-methoxy-4-(2-nitro-vinyl)-benzene; malonic acid dimethyl ester With sodium hydroxide In methanol at 0℃; for 0.75h; Stage #2: 4-fluoro-3-phenoxybenzaldehyde; formamide In methanol Reflux; | 63% |

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
1643-39-6
2-amino-4,6-di-tertbutylphenol

| Conditions | Yield |
|---|---|
| for 2h; Heating; | 62% |
-
-
3179-10-0
1-methoxy-4-(2-nitro-vinyl)-benzene

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
504-02-9
1,3-cylohexanedione

| Conditions | Yield |
|---|---|
| With piperidine; ammonium acetate In N,N-dimethyl-formamide at 20℃; for 7h; Michael Addition; Reflux; | 61% |

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
89765-17-3
3-(4-Ethoxyphenyl)-3-methyl-2-butanone

-
-
89765-18-4
1-(4-Fluoro-3-phenoxyphenyl)-4-(4-ethoxyphenyl)-4-methylpent-1-ene-3-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 20℃; for 6h; Condensation; | 60% |
-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
1530-32-1
ethyltriphenylphosphonium bromide

| Conditions | Yield |
|---|---|
| With potassium carbonate In 1,4-dioxane at 110℃; | 56.8% |
-
-
2033-24-1
cycl-isopropylidene malonate

-
-
3179-10-0
1-methoxy-4-(2-nitro-vinyl)-benzene

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: cycl-isopropylidene malonate; 1-methoxy-4-(2-nitro-vinyl)-benzene With triethylamine In ethanol at 20℃; for 3h; Michael Addition; Stage #2: 4-fluoro-3-phenoxybenzaldehyde With ammonium acetate In ethanol at 45℃; for 20h; Mannich Aminomethylation; | 55% |

-
-
34040-62-5
o-(chloromethyl)benzoic acid methyl ester

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With N1,N1,N12,N12-tetramethyl-7,8-dihydro-6H-dipyrido[1,2-a:2,1'-c][1,4]diazepine-2,12-diamine In N,N-dimethyl-formamide at 20℃; for 16h; Glovebox; Inert atmosphere; | 55% |
-
-
3179-10-0, 5576-97-6
(E)-1-methoxy-4-(2-nitrovinyl)benzene

-
-
68359-57-9
4-fluoro-3-phenoxybenzaldehyde

-
-
108-59-8
malonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: (E)-1-methoxy-4-(2-nitrovinyl)benzene; malonic acid dimethyl ester With sodium hydroxide In methanol at 0℃; for 0.75h; Stage #2: 4-fluoro-3-phenoxybenzaldehyde With ammonium acetate In methanol at 20 - 85℃; for 40h; | 53% |

4-Fluoro-3-phenoxybenzaldehyde Chemical Properties
IUPAC Name: 4-Fluoro-3-phenoxybenzaldehyde
Following is the structure of Benzaldehyde,4-fluoro-3-phenoxy- (CAS NO.68359-57-9):
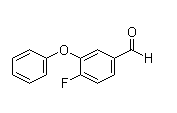
Empirical Formula: C13H9FO2
Molecular Weight: 216.2078
EINECS: 269-856-2
Index of Refraction: 1.591
Molar Refractivity: 59.44 cm3
Molar Volume: 175.8 cm3
Polarizability: 23.56 10-24cm3
Surface Tension: 43 dyne/cm
Density: 1.229 g/cm3
Flash Point: 142.5 °C
Enthalpy of Vaporization: 56.16 kJ/mol
Boiling Point: 320 °C at 760 mmHg
Vapour Pressure: 0.000327 mmHg at 25 °C
Product Categories: Aromatic Aldehydes & Derivatives (substituted);Aldehydes;C10 to C21;Carbonyl Compounds
Canonical SMILES: C1=CC=C(C=C1)OC2=C(C=CC(=C2)C=O)F
InChI: InChI=1S/C13H9FO2/c14-12-7-6-10(9-15)8-13(12)16-11-4-2-1-3-5-11/h1-9H
InChIKey: JDICMOLUAHZVDS-UHFFFAOYSA-N
4-Fluoro-3-phenoxybenzaldehyde Safety Profile
Hazard Codes:  Xn,
Xn, N
N
Risk Statements: 22-51/53
R22:Harmful if swallowed.
R51/53:Toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements: 61
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
RIDADR UN: 3082 9/PG 3
WGK Germany: 2
Hazard Note: Harmful
4-Fluoro-3-phenoxybenzaldehyde Specification
Benzaldehyde,4-fluoro-3-phenoxy- , its cas register number 68359-57-9. It also can be called 3-(Phenoxy)-4-fluoro-benzaldehyde ; and 4-Fluoro-3-phenoxybenzaldehyde .
Related Products
- 4-Fluoro-3-phenoxybenzaldehyde
- 68-36-0
- 683-60-3
- 6836-18-6
- 6836-19-7
- 6836-21-1
- 6836-22-2
- 68367-53-3
- 68368-37-6
- 6837-24-7
- 683-72-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View