-
Name
4-Hydroxy-3-methoxybenzyl alcohol
- EINECS 207-852-4
- CAS No. 498-00-0
- Article Data165
- CAS DataBase
- Density 1.226 g/cm3
- Solubility Soluble in 95% ethanol (5 %), water, and oils.
- Melting Point 110-117 ºC(lit.)
- Formula C8H10O3
- Boiling Point 313.1 ºC at 760 mmHg
- Molecular Weight 154.166
- Flash Point 143.2ºC
- Transport Information
- Appearance crystalline white to off-white powder
- Safety 26-36
- Risk Codes R36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Vanillylalcohol (6CI,8CI);3-Methoxy-4-hydroxybenzyl alcohol;4-(Hydroxymethyl)-2-methoxyphenol;4-Hydroxy-3-methoxybenzenemethanol;NSC 3993;V 0018;V 0018 (alcohol);Vanillicalcohol;Vanillin alcohol;p-(Hydroxymethyl)guaiacol;Benzenemethanol,4-hydroxy-3-methoxy-;
- PSA 49.69000
- LogP 0.89310
Synthetic route

-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With water | 100% |

| Conditions | Yield |
|---|---|
| With hydrogen In water at 70℃; Catalytic behavior; | A 99.9% B n/a |
| With hydrogen In water at 20 - 100℃; under 3750.38 Torr; for 1h; Kinetics; Reagent/catalyst; Temperature; Pressure; Autoclave; | A 90.9% B 9.1% |
| With 2 wt% Pd/C; hydrogen In water at 20 - 100℃; under 3750.38 Torr; for 1h; Kinetics; Reagent/catalyst; Autoclave; | A 21.8% B 78.3% |

| Conditions | Yield |
|---|---|
| With whole cells of recombinant strain VA1 In aq. phosphate buffer at 30℃; for 24h; pH=7.4; Temperature; Microbiological reaction; | 99.2% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol for 1h; | 98% |
| With aluminum oxide; zinc(II) tetrahydroborate In tetrahydrofuran at 20℃; for 0.08h; chemoselective reaction; | 97% |
| With water; nickel dichloride; zinc In N,N-dimethyl-formamide for 2h; Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; Trimethyl borate; dimethyl sulfate In tetrahydrofuran at 20℃; for 1.5h; | 98% |
| With diisopropoxytitanium(III) tetrahydroborate In dichloromethane for 4h; Ambient temperature; | 73% |
| With D-glucose In aq. phosphate buffer at 30℃; for 29h; pH=8; Enzymatic reaction; | 69% |
| With hydrogen In aq. phosphate buffer at 50℃; under 3750.38 Torr; for 24h; pH=2; Autoclave; |
-
-
1058649-06-1
2-methoxy-4-((methoxymethoxy)methyl) phenol

-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With 1-methylimidazole hydrogen sulfate at 120℃; for 0.025h; Microwave irradiation; chemoselective reaction; | 95% |
| phosphotungstic acid In ethanol for 4h; Heating; | 81% |

-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With silica gel In methanol at 60℃; for 3h; Inert atmosphere; | 95% |
-
-
86534-11-4
3-methoxy-4-(2-propenyloxy) benzenemethanol

-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With cerium(III) chloride; sodium iodide In acetonitrile for 6h; deallylation; Heating; | 94% |
-
-
1058649-10-7
4-((ethoxymethoxy)methyl)-2-methoxy phenol

-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With 1-methylimidazole hydrogen sulfate at 120℃; for 0.0333333h; Microwave irradiation; chemoselective reaction; | 93% |
| phosphotungstic acid In ethanol for 3h; Heating; | 91% |

-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| bismuth(lll) trifluoromethanesulfonate In tetrahydrofuran; water at 20℃; for 0.5h; | 90% |
-
-
121-33-5
vanillin

-
A
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
B
-
5533-03-9
2-methoxy-4-methoxymethylphenol

| Conditions | Yield |
|---|---|
| In methanol for 0.0833333h; Ambient temperature; | A n/a B 60% |
-
-
93-03-8
(3,4-dimethoxyphenyl)methanol

-
A
-
4383-06-6
3-hydroxy-4-methoxybenzyl alcohol

-
B
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With orcinol; Acetobacterium dehalogenans veratrol-O-demethylase; Desulfitobacterium hafniense methyltransferase dhaf4611 In aq. buffer at 35℃; for 24h; pH=6.5; Inert atmosphere; Enzymatic reaction; regioselective reaction; | A 47% B 53% |
-
-
121-33-5
vanillin

-
A
-
106-44-5
p-cresol

-
B
-
93-51-6
2-Methoxy-4-methylphenol

-
C
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With hydrogen In water at 100℃; under 15001.5 Torr; for 3h; Autoclave; | A 6.9% B 52.3% C 6.63% |
| With hydrogen In water at 100℃; under 15001.5 Torr; for 3h; Autoclave; | A 6.9% B 52.9% C 6.9% |
| With hydrogen In water at 100℃; under 15001.5 Torr; for 3h; Autoclave; | A 5.3% B 49.9% C 11.8% |


| Conditions | Yield |
|---|---|
| With ammonium chloride; magnesium at 20℃; for 3h; Irradiation; | A 8% B 51% |
| With sodium amalgam; ethanol man neutralisiert das Reaktionsprodukt mit H2SO4, filtriert und schuettelt das Filtrat mit Aether aus; | |
| With sodium amalgam; water | |
| With sodium amalgam; ethanol |

| Conditions | Yield |
|---|---|
| With Ximenia american In aq. phosphate buffer; water at 30℃; for 72h; pH=7; Enzymatic reaction; | A 51% B n/a |
| With roots of Conium maculatum In water at 20℃; for 48h; Enzymatic reaction; | A 27 %Chromat. B 73 %Chromat. |
-
-
33693-48-0
4-benzyloxy-3-methoxybenzyl alcohol

-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With Mortierella isabellina NRRL 1757 Ambient temperature; | 40% |
-
-
93-07-2
Veratric acid

-
A
-
3897-89-0
4-(hydroxymethyl)benzene-1,2-diol

-
B
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
C
-
93-03-8
(3,4-dimethoxyphenyl)methanol

| Conditions | Yield |
|---|---|
| With potassium hydroxide; samarium diiodide In tetrahydrofuran; water for 0.00194444h; Ambient temperature; | A 4% B 7% C 28% |

-
-
121-33-5
vanillin

-
A
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
B
-
121-34-6
3-methoxy-4-hydroxybenzoic acid

| Conditions | Yield |
|---|---|
| With Pseudomonas fluorescens B56 (IFO 12055) at 30℃; for 7h; Mechanism; growing conditions; | |
| With sodium hydroxide; formaldehyd; silver | |
| With sodium hydroxide; formaldehyd; silver |

| Conditions | Yield |
|---|---|
| With sodium hydroxide | |
| With sodium hydroxide |
-
-
495-76-1
piperonol

-
A
-
4383-06-6
3-hydroxy-4-methoxybenzyl alcohol

-
B
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In toluene for 3h; Heating; Title compound not separated from byproducts; |
-
-
20938-53-8
(+/-)-orientaline

-
A
-
72142-82-6
1,2-didehydrocoripallinium ion

-
B
-
450-14-6
6-methoxy-2-methyl-1,2,3,4-tetrahydro-isoquinolin-7-ol

-
C
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In methanol; water for 14h; Ambient temperature; ascorbate oxidase from Cucurbita pepo medullosa L., phosphate buffer pH 7.0; | A n/a B 29 mg C 43 mg D 62 mg |
| With dihydrogen peroxide In methanol; water for 14h; Product distribution; Ambient temperature; ascorbate oxidase from Cucurbita pepo medullosa L. or peroxidase from Nelumbo nucifera Gaertn.; phosphate buffer pH 7.0; | A n/a B 29 mg C 43 mg D 62 mg |

-
-
1196-92-5
Vanillylamin

-
A
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
B
-
121-34-6
3-methoxy-4-hydroxybenzoic acid

-
C
-
121-33-5
vanillin

| Conditions | Yield |
|---|---|
| With Pseudomonas fluorescens B56 (IFO 12055) at 30℃; for 7h; Mechanism; effect of growing and non-growing conditions, effect of reaction times; |

-
-
6971-51-3
3-methoxybenzyl alcohol

-
A
-
4383-05-5
2-hydroxy-3-methoxybenzyl alcohol

-
B
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction. Yields of byproduct given; |
-
-
75489-74-6
methyl 6-O-(4-hydroxy-3-methoxybenzyl)-α-D-glucopyranoside

-
A
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
B
-
97-30-3
methyl-alpha-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With benzoquinone N-chloroimine; water at 60℃; Rate constant; pH 1.1; |
-
-
69571-25-1
methyl 4-O-(4-hydroxy-3-methoxybenzyl)-α-D-glucopyranoside

-
A
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
B
-
97-30-3
methyl-alpha-D-glucopyranoside

| Conditions | Yield |
|---|---|
| With benzoquinone N-chloroimine; water at 60℃; Rate constant; pH 1.1; |
-
-
67-56-1
methanol

-
-
121-33-5
vanillin

-
A
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
B
-
5533-03-9
2-methoxy-4-methoxymethylphenol

| Conditions | Yield |
|---|---|
| for 0.0833333h; Ambient temperature; |


| Conditions | Yield |
|---|---|
| elektrolytische Reduktion an Quecksilber-Kathoden; |

| Conditions | Yield |
|---|---|
| elektrolytische Reduktion an Quecksilber-Kathoden; |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In acetonitrile at 40℃; for 8h; Catalytic behavior; Reagent/catalyst; | 100% |
| With palladium; oxygen; sodium hydrogencarbonate In water at 80℃; for 6h; Reagent/catalyst; | 100% |
| With titanium(IV) oxide; oxygen at 29.84℃; under 760.051 Torr; for 6h; Sealed tube; Irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 20℃; | 100% |
| toluene-4-sulfonic acid at 20℃; | 100% |
| With toluene-4-sulfonic acid | 99% |
-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
-
71-36-3
butan-1-ol

-
-
82654-98-6
4-(butoxymethyl)-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With Sn-MCM-41 zeolite at 100℃; for 4h; | 100% |
| With titanium cation-exchanged montmorillonite (Ti4+-mont) at 30℃; for 5h; Inert atmosphere; chemoselective reaction; | 98% |
| With sulfated tungstate at 80℃; for 3h; Green chemistry; chemoselective reaction; | 83% |

| Conditions | Yield |
|---|---|
| lipase PS-C "Amano" I In toluene at 40℃; for 22h; ceramic; Product distribution / selectivity; | 98% |
| Novozym 435 at 50℃; for 20 - 48h; Polyacrylate; Product distribution / selectivity; | 94.1% |
| Novozym 435 In hexane at 50℃; for 48h; Polyacrylate; Product distribution / selectivity; | 93.1% |

-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 15h; Reflux; | 98% |
-
-
64-17-5
ethanol

-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
-
13184-86-6
phenol, 4-(ethoxymethyl)-2-methoxy-

| Conditions | Yield |
|---|---|
| at 80℃; for 2h; Neat (no solvent); | 97% |

| Conditions | Yield |
|---|---|
| at 80℃; for 2h; Neat (no solvent); | 97% |
-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
-
100-39-0
benzyl bromide

-
-
33693-48-0
4-benzyloxy-3-methoxybenzyl alcohol

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In toluene Heating; | 96% |
| With potassium carbonate In methanol Inert atmosphere; Reflux; | 70.3% |
| With 18-crown-6 ether; potassium carbonate for 9h; Reflux; | |
| In methanol at 60℃; for 12h; |

| Conditions | Yield |
|---|---|
| 96% |
-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
-
106-95-6
allyl bromide

-
-
86534-11-4
3-methoxy-4-(2-propenyloxy) benzenemethanol

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 95% |
| With potassium carbonate In acetone at 60℃; | 93% |
| With potassium carbonate In acetone at 60℃; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-hydroxymethyl-2-methoxyphenol With ammonium formate In ethanol; water at 22℃; for 0.166667h; Stage #2: With formic acid In ethanol; water at 22℃; for 1h; Catalytic behavior; Reagent/catalyst; Temperature; Solvent; chemoselective reaction; | 95% |
| With lithium aluminium tetrahydride In tetrahydrofuran; chlorobenzene for 3h; Heating; | 87% |
| With triethylsilane; palladium dichloride In ethanol at 20℃; for 0.166667h; Inert atmosphere; | 96 %Chromat. |

| Conditions | Yield |
|---|---|
| at 80℃; for 2h; Neat (no solvent); | 95% |
| With alumina at 180℃; under 11251.1 Torr; for 0.666667h; Microwave irradiation; Sealed tube; | 63% |
-
-
109-87-5
Dimethoxymethane

-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
-
1058649-06-1
2-methoxy-4-((methoxymethoxy)methyl) phenol

| Conditions | Yield |
|---|---|
| With 12-tungstophosphoric acid immobilized on [bmim][FeCl4] at 75 - 82℃; for 0.00833333h; Microwave irradiation; | 95% |
| With 1-butyl-3-methylimidazolium tetrachloroindate for 0.0416667h; Microwave irradiation; chemoselective reaction; | 94% |
| phosphotungstic acid at 20℃; for 2h; | 93% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetone for 48h; Heating; | 94% |
| With potassium carbonate; potassium iodide In acetone for 48h; Heating / reflux; | 94% |

| Conditions | Yield |
|---|---|
| With pentafluorophenylboronic acid In toluene for 16h; Friedel-Crafts arylation; Reflux; Molecular sieve; | 94% |
-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
-
62-53-3
aniline

-
-
17696-53-6
N-(4-hydroxy-3-methoxybenzylidene)aniline

| Conditions | Yield |
|---|---|
| With CeO2 nanorods anchored on mesoporous carbon; air In toluene at 80℃; under 760.051 Torr; for 2h; | 94% |
| With TiO2 supported on MIL-101 framework, modified with CdS nanocrystals and decorated with co-catalytic Ni nanoparticles (Ni/CdS/TiO2-MIL-101) In acetonitrile at 27℃; for 48h; Inert atmosphere; Irradiation; | 72 %Chromat. |
| With γ-iron(III) oxide In toluene at 80℃; under 760.051 Torr; for 8h; | 81.2 %Chromat. |
-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
-
65439-58-9
2-phenyl-1-(2-thioxothiazolidin-3-yl)ethan-1-one

-
-
94475-58-8
2-methoxy-4-hydroxymethylphenyl phenylacetate

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 0.05h; Ambient temperature; | 93% |
-
-
462-95-3
formaldehyde diethyl acetal

-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
-
1058649-10-7
4-((ethoxymethoxy)methyl)-2-methoxy phenol

| Conditions | Yield |
|---|---|
| phosphotungstic acid at 20℃; for 3h; | 93% |
| With 1-butyl-3-methylimidazolium tetrachloroindate for 0.0416667h; Microwave irradiation; chemoselective reaction; | 93% |
| With 12-tungstophosphoric acid immobilized on [bmim][FeCl4] at 75 - 82℃; for 0.00833333h; Microwave irradiation; | 93% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone; toluene Reflux; | 93% |
| With tetrabutylammomium bromide; potassium carbonate In acetone Reflux; | |
| With potassium carbonate; potassium iodide In acetone at 75℃; for 8h; |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; boron trifluoride diethyl etherate; iodosylbenzene In dichloromethane at 20℃; for 0.5h; | 92% |
-
-
498-00-0
4-hydroxymethyl-2-methoxyphenol

-
-
75-36-5
acetyl chloride

-
-
57404-55-4
4-acetoxymethyl-2-methoxyphenol

| Conditions | Yield |
|---|---|
| With calcium oxide In 2-methyltetrahydrofuran at 20℃; for 6h; Green chemistry; chemoselective reaction; | 91% |
| In tetrahydrofuran at 60℃; for 0.5h; Microwave irradiation; | 100 %Spectr. |
4-Hydroxy-3-methoxybenzyl alcohol Chemical Properties
IUPAC Name: 4-(Hydroxymethyl)-2-methoxyphenol
Empirical Formula: C8H10O3
Molecular Weight: 154.1632g/mol
EINECS: 207-852-4
Structure of 4-Hydroxy-3-methoxybenzyl alcohol (CAS NO.498-00-0):
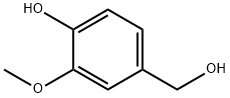
Index of Refraction: 1.57
Molar Refractivity: 41.26 cm3
Molar Volume: 125.6 cm3
Polarizability: 16.35×10-24cm3
Surface Tension: 48.9 dyne/cm
Density: 1.226 g/cm3
Flash Point: 143.2 °C
Enthalpy of Vaporization: 58.51 kJ/mol
Melting Point: 110-117 °C(lit.)
Boiling Point: 313.1 °C at 760 mmHg
Vapour Pressure: 0.000216 mmHg at 25 °C
Stability: Stable. Incompatible with strong oxidizing agents, strong acids.
Product Categories: Benzhydrols, Benzyl & Special Alcohols
Canonical SMILES: COC1=C(C=CC(=C1)CO)O
InChI: InChI=1S/C8H10O3/c1-11-8-4-6(5-9)2-3-7(8)10/h2-4,9-10H,5H2,1H3
InChIKey: ZENOXNGFMSCLLL-UHFFFAOYSA-N
4-Hydroxy-3-methoxybenzyl alcohol Safety Profile
Safety Information of 4-Hydroxy-3-methoxybenzyl alcohol (CAS NO.498-00-0):
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
WGK Germany: 3
Hazard Note: Irritant
4-Hydroxy-3-methoxybenzyl alcohol Specification
4-Hydroxy-3-methoxybenzyl alcohol , its cas register number is 498-00-0. It also can be called 4-Hydroxy-3-methoxybenzenemethanol ; 4-Hydroxy-3-methoxybenzyl alcohol ; 4-Hydroxy-3-methoxyphenylmethanol ; AI3-24186 ; Benzenemethanol, 4-hydroxy-3-methoxy- ; Vanillic alcohol ; Vanillin alcohol ; Vanillyl alcohol . 4-Hydroxy-3-methoxybenzyl alcohol (CAS NO.498-00-0) is crystalline white to off-white powder .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View