-
Name
4-(METHYLAMINO)-3-NITROBENZOIC ACID
- EINECS 609-904-5
- CAS No. 41263-74-5
- Article Data38
- CAS DataBase
- Density 1.472 g/cm3
- Solubility
- Melting Point >300 °C
- Formula C8H8N2O4
- Boiling Point 393.7 °C at 760 mmHg
- Molecular Weight 196.163
- Flash Point 191.9 °C
- Transport Information
- Appearance Yellow solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 4-(Methylamino)-3-nitrobenzoicacid;NSC 138300;
- PSA 95.15000
- LogP 1.93090
Synthetic route

| Conditions | Yield |
|---|---|
| In water at 100℃; for 14h; | 100% |
| In water at 80℃; for 5h; | 100% |
| Stage #1: methylamine; 4-chloro-3-nitrobenzoate In water for 4h; Reflux; Stage #2: With sulfuric acid In water at 20℃; pH=2; Product distribution / selectivity; | 95.5% |
-
-
403-21-4
3-fluoro-4-nitrobenzoic acid

-
-
74-89-5
methylamine

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluoro-4-nitrobenzoic acid; methylamine In DMF (N,N-dimethyl-formamide); water at 20℃; for 1h; Stage #2: With potassium hydrogensulfate In water pH=2; | 100% |
-
-
453-71-4
3-nitro-4-fluorobenzoic acid

-
-
74-89-5
methylamine

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| In methanol; ethanol at 20℃; for 2h; | 99% |
| In methanol at 20℃; for 18h; | 99% |
| In methanol at 20℃; for 10h; | 98% |
-
-
6319-40-0
4-bromo-3-nitrobenzoic acid

-
-
74-89-5
methylamine

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With D-myo-inositol; copper; caesium carbonate In water at 100℃; | 92% |
-
-
96-99-1
4-chloro-3-nitrobenzoate

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With methylamine In water | 86% |
-
-
36242-50-9
4-methylamino-3-nitro-benzoic acid methyl ester

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-methylamino-3-nitro-benzoic acid methyl ester With lithium hydroxide In tetrahydrofuran; water at 20℃; for 16h; Stage #2: With hydrogenchloride In tetrahydrofuran; water pH=Ca. 5; | 84% |
| With lithium hydroxide In tetrahydrofuran; water at 20℃; for 16h; | 84% |
| With water; lithium hydroxide In tetrahydrofuran at 20℃; for 16h; | 84% |

| Conditions | Yield |
|---|---|
| Stage #1: 3,4-dinitrobenzoic acid; methylamine With triethylamine In methanol; ethanol at 20℃; for 24h; Stage #2: With acetic acid In water | 81% |
-
-
591-09-3
nitro acetate

-
-
28096-56-2
4-(N,N-dimethylamino)-3-nitrobenzoic acid

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid
-
-
71254-71-2
ethyl 4-(methylamino)-3-nitrobenzoate

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
28096-56-2
4-(N,N-dimethylamino)-3-nitrobenzoic acid

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate; sodium carbonate | |
| With nitrosyl acetate |
-
-
59935-39-6
4-dimethylamino-3-nitrobenzaldehyde

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate; sodium carbonate | |
| Multi-step reaction with 4 steps 1: alkali; hydroxylamine hydrochloride 2: acetic acid anhydride; sodium acetate 3: NaOH-solution; alcohol 4: potassium permanganate; natrium carbonate View Scheme |
-
-
7664-93-9
sulfuric acid

-
-
82080-46-4
4-(methoxycarbonyl)-2-nitro-N,N-dimethylaniline

-
-
7697-37-2
nitric acid

-
A
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| at 70 - 75℃; |

-
-
19005-63-1
4-(dimethylamino)-3-nitrobenzonitrile

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NaOH-solution; alcohol 2: potassium permanganate; natrium carbonate View Scheme |

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: acetic acid anhydride; sodium acetate 2: NaOH-solution; alcohol 3: potassium permanganate; natrium carbonate View Scheme |
-
-
619-84-1
p-N,N-dimethylaminobenzoic acid

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: HNO3+H2SO4 / 0 °C 2: potassium permanganate; natrium carbonate View Scheme |
-
-
937625-32-6
ethyl 4-ethoxy-3-nitrobenzoate

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: alcohol / 130 - 140 °C / im Druckrohr 2: KOH-solution View Scheme |
-
-
14719-83-6
methyl 3-nitro-4-chlorobenzoate

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: N,N-dimethyl-formamide / 70 - 90 °C / Inert atmosphere 2.1: lithium hydroxide / tetrahydrofuran; water / 16 h / 20 °C 2.2: pH Ca. 5 View Scheme | |
| Multi-step reaction with 2 steps 1: tetrahydrofuran; N,N-dimethyl-formamide / 20 - 90 °C / Inert atmosphere 2: lithium hydroxide / tetrahydrofuran; water / 16 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: N,N-dimethyl-formamide; tetrahydrofuran / 70 - 90 °C 2: lithium hydroxide; water / tetrahydrofuran / 16 h / 20 °C View Scheme |
-
-
456-22-4
4-Fluorobenzoic acid

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sulfuric acid; nitric acid / 10 h / 20 °C 2: methanol / 10 h / 20 °C View Scheme |
-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

-
-
82357-48-0
4-methylamino-3-nitro-benzoic acid chloride

| Conditions | Yield |
|---|---|
| With thionyl chloride Chlorination; Heating; | 100% |
| With thionyl chloride; N,N-dimethyl-formamide In dichloromethane Reflux; | 100% |
| With thionyl chloride; N,N-dimethyl-formamide In dichloromethane at 30℃; | 100% |
-
-
309956-78-3
(R)-piperidin-3-ylcarbamic acid tert-butyl ester

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

-
-
1549812-10-3
1,1-dimethylethyl ((3R)-1-{[4-(methylamino)-3-nitrophenyl]carbonyl}-3-piperidinyl)carbamate

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 16h; | 100% |
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 16h; | 100% |
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 16h; | 100% |
-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

-
-
66315-15-9
3-amino-4-(methylamino)benzoic acid

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol at 20℃; for 2h; | 99% |
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; | 99% |
| With palladium on activated charcoal; hydrogen In methanol at 20℃; for 48h; | 36.5% |
-
-
103041-38-9
2-chloromethyl-1-methyl-1H-benzimidazole-5 ethyl acetate

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

-
-
1187067-68-0
ethyl 3-[[4-(methylamino)-3-nitrobenzoyl](pyridin-2-yl)amino]propanoate hydrochloride

| Conditions | Yield |
|---|---|
| With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 18h; Solvent; Reagent/catalyst; | 95.2% |
-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| With thionyl chloride In N,N-dimethyl-formamide; toluene at 0 - 70℃; | 82% |
-
-
67-56-1
methanol

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

-
-
36242-50-9
4-methylamino-3-nitro-benzoic acid methyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid for 6h; Reflux; | 77% |
| Stage #1: methanol; 4-(methylamino)-3-nitrobenzoic acid With hydrogenchloride In N,N-dimethyl-formamide for 10h; Reflux; Stage #2: With sodium hydrogencarbonate In water | 32% |
-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

-
-
106-40-1
4-bromo-aniline

-
-
1003098-13-2
N-(4-bromo-phenyl)-4-methylamino-3-nitro-benzamide

| Conditions | Yield |
|---|---|
| Stage #1: 4-(methylamino)-3-nitrobenzoic acid With pyridine; 1-chloro-1-(dimethylamino)-2-methyl-1-propene In dichloromethane Stage #2: 4-bromo-aniline In dichloromethane for 16h; | 68% |
-
-
64-17-5
ethanol

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

-
-
66315-15-9
3-amino-4-(methylamino)benzoic acid

| Conditions | Yield |
|---|---|
| palladium-carbon | 61% |
-
-
103041-38-9
2-chloromethyl-1-methyl-1H-benzimidazole-5 ethyl acetate

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-(methylamino)-3-nitrobenzoic acid With thionyl chloride In N,N-dimethyl-formamide for 0.75h; Reflux; Stage #2: 2-chloromethyl-1-methyl-1H-benzimidazole-5 ethyl acetate With triethylamine In tetrahydrofuran at 20 - 60℃; Stage #3: With hydrogenchloride In water; ethyl acetate Product distribution / selectivity; | 47.2% |
-
-
103041-38-9
2-chloromethyl-1-methyl-1H-benzimidazole-5 ethyl acetate

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-(methylamino)-3-nitrobenzoic acid With thionyl chloride In N,N-dimethyl-formamide for 0.75h; Reflux; Stage #2: 2-chloromethyl-1-methyl-1H-benzimidazole-5 ethyl acetate With triethylamine In tetrahydrofuran at 20 - 60℃; Stage #3: With hydrogenchloride In ethyl acetate Product distribution / selectivity; | 47.2% |
-
-
64-17-5
ethanol

-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

-
-
71254-71-2
ethyl 4-(methylamino)-3-nitrobenzoate

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

-
-
108-24-7
acetic anhydride

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: SOCl2 2: NH3 3: H2 / Pd/C / ethanol 4: 65 percent / Na2S2O5 / dimethylformamide / 100 °C View Scheme |
-
-
41263-74-5
4-(methylamino)-3-nitrobenzoic acid

-
-
39033-67-5
3-amino-4-(methylamino)benzamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: SOCl2 2: NH3 3: H2 / Pd/C / ethanol View Scheme |
4-Methylamino-3-nitrobenzoic acid Chemical Properties
Product Name: 4-Methylamino-3-nitrobenzoic acid (CAS NO.41263-74-5)
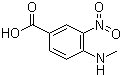
Molecular Formula: C8H8N2O4
Molecular Weight: 196.16g/mol
Mol File: 41263-74-5.mol
Boiling point: 393.7 °C at 760 mmHg
Flash Point: 191.9 °C
Density: 1.472 g/cm3
Index of Refraction: 1.662
Molar Refractivity: 49.33 cm3
Molar Volume: 133.2 cm3
Surface Tension: 68.7 dyne/cm
Enthalpy of Vaporization: 67.88 kJ/mol
Vapour Pressure: 6.62E-07 mmHg at 25°C
XLogP3-AA: 1.9
H-Bond Donor: 2
H-Bond Acceptor: 5
Structure Descriptors of 4-Methylamino-3-nitrobenzoic acid (CAS NO.41263-74-5):
IUPAC Name: 4-(methylamino)-3-nitrobenzoic acid
Canonical SMILES: CNC1=C(C=C(C=C1)C(=O)O)[N+](=O)[O-]
InChI: InChI=1S/C8H8N2O4/c1-9-6-3-2-5(8(11)12)4-7(6)10(13)14/h2-4,9H,1H3,(H,11,12)
InChIKey: KSMLIIWEQBYUKA-UHFFFAOYSA-N
Related Products
- 4-Methylamino-3-nitrobenzoic acid
- 41263-94-9
- 41264-06-6
- 41266-78-8
- 4126-70-9
- 41267-76-9
- 41272-40-6
- 41274-09-3
- 41276-30-6
- 4127-63-3
- 41276-95-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View