-
Name
4-Nitrophenol
- EINECS 202-811-7
- CAS No. 100-02-7
- Article Data2114
- CAS DataBase
- Density 1.395 g/cm3
- Solubility 1.6 g/100 mL (25 °C) in water
- Melting Point 112-114 °C
- Formula C6H5NO3
- Boiling Point 279 °C at 760 mmHg
- Molecular Weight 139.111
- Flash Point 141.9 °C
- Transport Information UN 1663 6.1/PG 3
- Appearance Yellow to brown crystals
- Safety 28-28A-45-36/37-16-7
- Risk Codes 20/21/22-33-39/23/24/25-23/24/25-11
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, T,
T, F
F
- Synonyms Phenol,p-nitro- (8CI);1-Hydroxy-4-nitrobenzene;4-Hydroxy-1-nitrobenzene;4-Hydroxynitrobenzene;Phenol, 4-nitro-;Niphen;p-Hydroxynitrobenzene;p-Nitrophenol;
- PSA 66.05000
- LogP 1.82360
Synthetic route

| Conditions | Yield |
|---|---|
| With aminomethyl resin-supported N-propylbarbituric acid; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran at 20℃; | 100% |
| With chloro-trimethyl-silane; sodium cyanoborohydride In acetonitrile at 20℃; for 0.25h; ether cleavage; | 95% |
| With sodium tetrahydroborate; tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran for 1h; | 94% |

| Conditions | Yield |
|---|---|
| sodium hydrogen sulfate; silica gel In dichloromethane at 20℃; for 1h; | 100% |
| With diphosphorus tetraiodide In dichloromethane at 0℃; for 0.75h; | 92% |
| With bismuth(III) chloride In water; acetonitrile at 50℃; for 3h; | 92% |
| With Montmorillonite K 10 In benzene at 50℃; for 72h; | 20% |
-
-
133754-19-5
trans-2-(p-nitrophenoxy)-6-carboxytetrahydropyran

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium chloride at 50℃; Rate constant; Mechanism; var. pH; other acetals; other solvent; rate constant vs. pH; | 100% |

| Conditions | Yield |
|---|---|
| silica gel; toluene-4-sulfonic acid In water; toluene at 80℃; for 8h; | 100% |
| With ammonium acetate In methanol at 20℃; for 2h; | 99% |
| With Vigna unguiculata powder In water; isopropyl alcohol at 30℃; for 72h; | 99% |
-
-
13165-89-4
4-nitrophenyl methylsulphonylmethanesulphonate

-
-
100-46-9
benzylamine

-
A
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With pH 13 In water at 25℃; | A n/a B 100% |
| With potassium hydroxide at 25℃; Rate constant; also with benzylamine buffers; var. conc.; |


-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 20℃; for 24h; | 100% |
-
-
13303-10-1
tert-Butyl 4-nitrophenyl carbonate

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With erbium(III) triflate In ethanol for 25h; Microwave irradiation; | 100% |
| With methanol; carbon tetrabromide; triphenylphosphine for 12h; Reflux; | 92% |
| With zinc diacetate; water-d2; N-ethyl-N,N-diisopropylamine; tris(2-benzylaminoethyl)amine In dimethylsulfoxide-d6 at 21.84℃; Kinetics; Reagent/catalyst; |
-
-
24067-17-2
4-nitrophenylboronic acid

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With water; oxygen; sodium sulfite at 50℃; for 1h; Green chemistry; | 100% |
| With N-ethyl-N,N-diisopropylamine In water; acetonitrile at 20℃; for 30h; Irradiation; Green chemistry; | 99% |
| With N-ethyl-N,N-diisopropylamine In water; acetonitrile for 48h; Irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide at 150℃; for 0.1h; Autoclave; | 100% |
| Stage #1: 4-nitro-aniline With tetrafluoroboric acid In water at 20℃; for 0.0333333h; Stage #2: With sodium nitrite In water at 0℃; for 0.5h; Stage #3: With copper(I) oxide; copper(II) sulfate In water at 0 - 20℃; for 0.5h; | 87% |
| Stage #1: 4-nitro-aniline With sulfuric acid In water Stage #2: With sulfuric acid; sodium nitrite In water at 0 - 5℃; for 0.166667h; Heating; | 60% |

| Conditions | Yield |
|---|---|
| With arylsulfate sulfotransferase from Desulfitobacterium hafniense In acetone at 30℃; for 96h; pH=9; Kinetics; pH-value; Green chemistry; Enzymatic reaction; regioselective reaction; | A n/a B 100% |


| Conditions | Yield |
|---|---|
| With β-D-glucose; copper(II) acetate monohydrate; potassium hydroxide In water; dimethyl sulfoxide at 20 - 120℃; for 24h; | 99% |
| With tetra(n-butyl)ammonium hydroxide; water at 100℃; for 4h; | 96% |
| Stage #1: 4-chlorobenzonitrile With sodium hydroxide In water at 170℃; under 3750.38 Torr; for 8h; Inert atmosphere; Stage #2: With hydrogenchloride In water at 80℃; for 1h; pH=1.5; Temperature; pH-value; Pressure; Reagent/catalyst; | 96.7% |

| Conditions | Yield |
|---|---|
| Stage #1: para-nitrophenyl bromide With potassium hydroxide; tris-(dibenzylideneacetone)dipalladium(0); 2-((di-adamantan-1-yl)phosphaneyl)-1-(2,6-diisopropylphenyl)-1H-imidazole In 1,4-dioxane; water at 100℃; for 20h; Inert atmosphere; Stage #2: With hydrogenchloride In 1,4-dioxane; water at 20℃; Inert atmosphere; | 99% |
| With β-D-glucose; copper(II) acetate monohydrate; potassium hydroxide In water; dimethyl sulfoxide at 20 - 120℃; for 24h; | 99% |
| With dicyclohexyl-(2′,4′,6′-triisopropyl-3,6-dimethoxy-[1,1′-biphenyl]-2-yl)phosphine; boric acid; palladium diacetate; caesium carbonate In 1-methyl-pyrrolidin-2-one at 80℃; for 24h; Schlenk technique; Inert atmosphere; | 99% |
-
-
198829-77-5
1-[(2-methoxyethoxy)methoxy]-4-nitrobenzene

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| sodium hydrogen sulfate; silica gel In dichloromethane at 20℃; for 1.5h; | 99% |
| With diphosphorus tetraiodide In dichloromethane 0 degC, 25 min and room temp., 5 min; | 92% |

| Conditions | Yield |
|---|---|
| With 2-(methylsulfonyl)ethyl alcohol; sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; | 99% |
| With sodium hydroxide In dimethyl sulfoxide at 80℃; for 12h; | 90% |
| With methyl propargyl alcohol; potassium tert-butylate In dimethyl sulfoxide at 125℃; for 0.0333333h; microwave irradiation; | 78% |

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide at 20℃; for 0.5h; | 99% |
-
-
17763-80-3
para-nitrophenyl triflate

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With tetraethylammonium hydroxide In 1,4-dioxane at 20℃; for 1h; | 99% |

| Conditions | Yield |
|---|---|
| With β-D-glucose; copper(II) acetate monohydrate; potassium hydroxide In water; dimethyl sulfoxide at 20 - 120℃; for 16h; | 99% |
| With copper(I) oxide; N-phenylpicolinamide; sodium hydroxide In water; dimethyl sulfoxide at 160℃; for 0.166667h; Microwave irradiation; | 98% |
| With basolite C300; potassium hydroxide In water; dimethyl sulfoxide at 125℃; for 12h; | 96% |
-
-
20443-91-8
2-(4-nitrophenoxy)tetrahydropyran

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With montmorillonite K-10 In methanol at 40 - 50℃; for 0.4h; | 98% |
| With methanol; zirconium(IV) chloride at 20℃; for 5h; | 86% |
| With acid-washed bentonite In acetone at 40 - 50℃; for 0.333333h; | 86.7% |

| Conditions | Yield |
|---|---|
| With lithium chloride In N,N-dimethyl-formamide for 24h; Heating; | 98% |
| With water; hydrogen bromide; Aliquat 336 at 105℃; for 3.5h; Catalytic behavior; | 97% |
| With copper(I) oxide; sodium methylate In methanol at 185℃; for 12h; Autoclave; | 87% |
-
-
117635-44-6
tert-butyldimethyl(4-nitrophenoxy)silane

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With water; potassium carbonate In ethanol at 75℃; for 2h; | 98% |
| With triethylamine N-oxide In methanol for 0.5h; | 96% |
| With hafnium tetrakis(trifluoromethanesulfonate) In methanol at 20℃; for 10h; | 96% |

| Conditions | Yield |
|---|---|
| With cerium(III) chloride; sodium iodide In acetonitrile for 4h; tosylate cleavage; Heating; | 98% |
| With tetraethylammonium hydroxide In 1,4-dioxane at 20℃; for 24h; | 92% |
| With potassium fluoride on basic alumina for 0.1h; Substitution; microwave irradiation; | 86% |
-
-
1093198-55-0
C22H15NO5

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With (triphenylphosphine)gold(I) chloride; silver trifluoromethanesulfonate In ethanol; benzene at 20℃; for 0.5h; | 98% |
-
-
1080-04-2
p-nitrophenyl sulfate

-
-
58822-25-6
Leu-enkephalin

-
A
-
100-02-7
4-nitro-phenol

-
B
-
80632-52-6
sulfated [Leu5]-enkephalin

| Conditions | Yield |
|---|---|
| With arylsulfate sulfotransferase from Desulfitobacterium hafniense In aq. buffer at 30℃; for 144h; pH=8; Green chemistry; Enzymatic reaction; regioselective reaction; | A n/a B 98% |


| Conditions | Yield |
|---|---|
| With methylene blue; N-ethyl-N,N-diisopropylamine In water; acetonitrile at 20℃; for 7h; Schlenk technique; Irradiation; | 98% |
-
-
83235-15-8
4-(4'-Nitrophenoxy)-2,3,5,6-tetrafluoropyridine

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With potassium fluoride; 18-crown-6 ether; Methyl thioglycolate In water; acetonitrile at 50℃; for 2h; | 98% |
-
-
171364-83-3
4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)nitrobenzene

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With [Rh2(bpy)2(μ-OAc)2(OAc)2]; oxygen; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide under 760.051 Torr; for 18h; Irradiation; | 98% |
| With dihydrogen peroxide In ethanol at 20℃; for 0.5h; pH=9.2; |
-
-
20455-07-6
p-nitrophenyl methanesulfonate

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With tetraethylammonium hydroxide In 1,4-dioxane at 20℃; for 3h; | 97% |
| With sodium azide; copper(ll) sulfate pentahydrate; water; sodium carbonate; sodium L-ascorbate; L-proline In dimethyl sulfoxide at 70℃; for 24h; | 71% |
-
-
86497-88-3
4<(2-methyl-2-propenyl)oxy>-1-nitrobenzene

-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 20℃; for 24h; | 97% |

-
A
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With Dess-Martin periodane In chloroform-d1 for 0.333333h; | A 87% B 97% |

| Conditions | Yield |
|---|---|
| With triethylamine In water; acetonitrile at 25℃; for 7h; Kinetics; Reagent/catalyst; | A n/a B 96% |
| With triethylamine hydrochloride; triethylamine In water; acetonitrile at 25℃; Rate constant; different Et3N concentrations and compositions of solvent mixtures; | |
| With sodium ethanolate In ethanol at 25℃; Kinetics; Further Variations:; Reagents; Elimination; |
-
-
1189-71-5
isocyanate de chlorosulfonyle

-
-
100-02-7
4-nitro-phenol

-
-
89692-65-9
N-<<(4-nitrophenyl)oxy>carbonyl>sulfamyl chloride

| Conditions | Yield |
|---|---|
| In diethyl ether for 2h; | 100% |
| With benzene | |
| In benzene | |
| In dichloromethane at 20℃; for 1.5h; | |
| With benzene |

| Conditions | Yield |
|---|---|
| With benzyltriphenylphosphonium peroxodisulfate; potassium bromide In acetonitrile for 8.5h; Heating; | 100% |
| With N-benzyl-N,N-dimethyl anilinium peroxodisulfate; potassium bromide In acetonitrile for 8h; Reflux; regioselective reaction; | 97% |
| With N-Bromosuccinimide; fluorosulphonic acid In acetonitrile at 20℃; for 48h; | 95% |

| Conditions | Yield |
|---|---|
| With copper(I) chloride; potassium borohydride In methanol for 0.166667h; Ambient temperature; | 100% |
| With palladium diacetate; carbon monoxide; triphenylphosphine In water; acetic acid at 56℃; under 532 Torr; for 14h; | 100% |
| With hydrazine hydrate In ethanol at 80℃; | 100% |

| Conditions | Yield |
|---|---|
| K5 In acetonitrile at 20℃; for 0.333333h; | 100% |
| With SBA-15-Ph-Pr-SO3H at 20℃; for 0.833333h; | 100% |
| With magnesium(II) perchlorate at 20℃; for 1.5h; | 99% |

| Conditions | Yield |
|---|---|
| With pyridine at 0 - 20℃; Inert atmosphere; | 100% |
| With 4-(dimethylamino)pyridine hydrochloride In toluene at 110℃; for 6h; | 98% |
| With sodium hydride In tetrahydrofuran at 20℃; for 1h; | 97% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 0.75h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; | 98% |
| With triethylamine In ethyl acetate at 0 - 20℃; for 0.166667h; Green chemistry; | 97% |
-
-
100-02-7
4-nitro-phenol

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
1153-45-3
4-nitrophenyl 4-methylbenzenesulfonate

| Conditions | Yield |
|---|---|
| Stage #1: 4-nitro-phenol; p-toluenesulfonyl chloride With potassium carbonate In acetone at 20 - 25℃; for 2.5h; Stage #2: With hydrogenchloride In water; acetone | 100% |
| With triethylamine In dichloromethane at 20℃; for 24h; Inert atmosphere; | 99% |
| With potassium carbonate for 0.0833333h; microwave irradiation; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 1h; Ambient temperature; | 100% |
| With triethylamine In tetrahydrofuran at 0℃; for 1h; | 100% |
| In 1,4-dioxane; pyridine for 2h; Ambient temperature; | 83% |
| With iodine; magnesium; benzene |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 1h; Ambient temperature; | 100% |
| With triethylamine In tetrahydrofuran at 0℃; for 1h; | 100% |
| In 1,4-dioxane; pyridine for 2h; Ambient temperature; | 65% |
| With iodine; magnesium; benzene |

| Conditions | Yield |
|---|---|
| for 20h; Heating; | 100% |
| for 16h; Heating; |

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether for 0.5h; Ambient temperature; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether at 0℃; for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether at 0℃; for 0.5h; | 100% |
-
-
100-02-7
4-nitro-phenol

-
-
15562-09-1
fluorodinitroacetonitrile

-
-
75767-62-3
p-nitrophenyl fluorodinitroacetimidate

| Conditions | Yield |
|---|---|
| In diethyl ether; dichloromethane at 60℃; under 7500600 Torr; for 55h; | 100% |
-
-
100-02-7
4-nitro-phenol

-
-
1161-13-3
N-Cbz-L-Phe

-
-
2578-84-9
N-benzyloxycarbonyl-L-phenylalanine p-nitrophenyl ester

| Conditions | Yield |
|---|---|
| With N,N'-Bis(2-oxo-3-oxazolidinyl)phosphorodiamidic azide; triethylamine In dichloromethane | 100% |
| With pyridine; 2,6-di-tert-butyl-4-methyl-phenol In benzene for 12h; | 81% |
| With pyridine; diphenyl hydrogen phosphite; mercury dichloride |
-
-
100-02-7
4-nitro-phenol

-
-
56512-49-3
dabsyl chloride

-
-
146303-71-1
4-(4-Dimethylamino-phenylazo)-benzenesulfonic acid 4-nitro-phenyl ester

| Conditions | Yield |
|---|---|
| With carbonate-bicarbonate buffer In acetone; acetonitrile 1.) 15 min, 2.) reflux; | 100% |
| With carbonate-bicarbonate buffer In acetone for 0.5h; Heating; |
-
-
100-02-7
4-nitro-phenol

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In 1,4-dioxane | 100% |
-
-
100-02-7
4-nitro-phenol

-
-
89760-77-0
3-<(benzyloxycarbonyl)aminomethyl>benzoic acid

-
-
89760-78-1
p-nitrophenyl 3-<(benzyloxycarbonyl)aminomethyl>benzoate

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide | 100% |
-
-
100-02-7
4-nitro-phenol

-
-
90889-43-3
<2,6-2H2>-4-nitrophenol

| Conditions | Yield |
|---|---|
| With water-d2; sulfuric acid-d2 at 120℃; for 48h; | 100% |
| With water-d2; hydrogen chloride at 175℃; for 0.333333h; Microwave irradiation; | 82% |
| With water-d2; hydrogen chloride for 90h; Heating; | 2.1 g |
| With sulfuric acid-d2 at 120℃; sealed tube; |
-
-
100-02-7
4-nitro-phenol

-
-
14609-74-6
p-nitrophenolate

| Conditions | Yield |
|---|---|
| With NaH-cryptand<2.2.1) In tetrahydrofuran for 0.00833333h; other reagents, other times, other solvent, other yields; | 100% |
| With NaH-cryptand<2.2.1> In tetrahydrofuran for 0.00833333h; | 100% |
| With N-butylamine In dimethyl sulfoxide; benzene at 25℃; Equilibrium constant; ionization in solvent mixtures with different ratio; |
-
-
100-02-7
4-nitro-phenol

-
-
1464-12-6
2-(4-(benzyloxy)-1H-indol-3-yl)acetic acid

-
-
144923-56-8
4-Nitrophenyl <(4-Benzyloxy)-1H-indol-3-yl>acetate

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane for 1h; Ambient temperature; | 100% |
| With dicyclohexyl-carbodiimide In ethyl acetate 1) ice-bath, 1 h, 2) r.t., 18 h; | 57.7% |
-
-
100-02-7
4-nitro-phenol

-
-
107-30-2
chloromethyl methyl ether

-
-
880-03-5
1-(methoxymethoxy)-4-nitrobenzene

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; Cooling with ice; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; | 89% |
| (i) NaOEt, EtOH, toluene, (ii) /BRN= 505943/; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With t-butyl bromide; triethylamine at 35℃; for 24h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone; toluene for 24h; Williamson Ether Synthesis; Reflux; | 100% |
| Stage #1: 4-nitro-phenol With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.5h; Inert atmosphere; Stage #2: propargyl bromide In N,N-dimethyl-formamide at 20℃; for 10h; Inert atmosphere; | 100% |
| Stage #1: 4-nitro-phenol With potassium carbonate In acetonitrile at 20℃; for 0.166667h; Stage #2: propargyl bromide In acetonitrile Reflux; | 100% |
-
-
100-02-7
4-nitro-phenol

-
-
15933-59-2
1,1,3,3-tetramethyldisilazane

-
-
79516-20-4
Dimethyl-(4-nitro-phenoxy)-silane

| Conditions | Yield |
|---|---|
| 100% | |
| at 20 - 160℃; for 2h; Inert atmosphere; |
4-Nitrophenol Consensus Reports
EPA Genetic Toxicology Program. Community Right-To-Know List. Reported in EPA TSCA Inventory.
4-Nitrophenol Specification
The 4-Nitrophenol with CAS registry number of 100-02-7 is also known as Phenol, 4-nitro-. The IUPAC name and product name are the same. It belongs to product categories of Organics; Phenoles and thiophenoles; Analytical Chemistry; Biochemistry; Coupling Reactions (Peptide Synthesis); Indicator (pH); Peptide Synthesis; pH Indicators. Its EINECS registry number is 202-811-7. In addition, the formula is C6H5NO3 and the molecular weight is 139.11. This chemical is a yellow to brown crystal and should be sealed in ventilated, cool room away from fire and heat. What's more, this chemical is soluble in sodium carbonate, sodium hydroxide solution, and other organic solvents such as ethanol, ether, chloroform and benzene.
Physical properties about 4-Nitrophenol are: (1)ACD/LogP: 1.67; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.66; (4)ACD/LogD (pH 7.4): 1.272; (5)ACD/BCF (pH 5.5): 10.707; (6)ACD/BCF (pH 7.4): 4.385; (7)ACD/KOC (pH 5.5): 188.961; (8)ACD/KOC (pH 7.4): 77.385; (9)#H bond acceptors: 4; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 2; (12)Index of Refraction: 1.612; (13)Molar Refractivity: 34.68 cm3; (14)Molar Volume: 99.705 cm3; (15)Surface Tension: 60.24 dyne/cm; (16)Density: 1.395 g/cm3; (17)Flash Point: 141.896 °C; (18)Enthalpy of Vaporization: 53.846 kJ/mol; (19)Boiling Point: 278.999 °C at 760 mmHg; (20)Vapour Pressure: 0.002 mmHg at 25 °C.
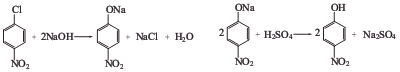
Preparation of 4-Nitrophenol: it is prepared by hydrolysis reaction of chloronitrobenzene.
Uses of 4-Nitrophenol: it is used as an intermediate in the synthesis of paracetamol and also is used for the preparation of phenetidine or acetophenetidine, indicators and raw materials for fungicides. What's more, it is used to produce methyl-phosphonic acid bis-(4-nitro-phenyl ester) by reaction with methyl-phosphonic acid dichloride. The reaction occurs with reagent Et3N and solvent CH2Cl2 at 20 °C for 1 hour. The yield is about 52%.

When you are using this chemical, please be cautious about it. As a chemical, it is harmful and danger of very serious irreversible effects through inhalation, in contact with skin and if swallowed. Besides, it is highly flammable and has danger of cumulative effects. During using it, wear suitable protective clothing and gloves. Keep away from sources of ignition. After using it, keep container tightly closed. If contact with skin, wash immediately. In case of accident or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC(=CC=C1[N+](=O)[O-])O
2. InChI: InChI=1S/C6H5NO3/c8-6-3-1-5(2-4-6)7(9)10/h1-4,8H
3. InChIKey: BTJIUGUIPKRLHP-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LD50 | unreported | 150mg/kg (150mg/kg) | "Chemistry of Pesticides," Melnikov, N.N., New York, Springer-Verlag New York, Inc., 1971Vol. -, Pg. 97, 1971. | |
| dog | LDLo | intravenous | 10mg/kg (10mg/kg) | U.S. Public Health Service, Public Health Bulletin. Vol. 271, Pg. 131, 1941. | |
| frog | LDLo | subcutaneous | 60mg/kg (60mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: ATAXIA | Revue Medicale de la Suisse Romande. Vol. 16, Pg. 449, 1896. |
| guinea pig | LD50 | skin | > 1gm/kg (1000mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | National Technical Information Service. Vol. OTS0570918, |
| guinea pig | LDLo | subcutaneous | 200mg/kg (200mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: COMA | Revue Medicale de la Suisse Romande. Vol. 16, Pg. 449, 1896. |
| mammal (species unspecified) | LD50 | oral | 247mg/kg (247mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 45(10), Pg. 16, 1980. | |
| mammal (species unspecified) | LD50 | skin | 920mg/kg (920mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 45(10), Pg. 16, 1980. | |
| mammal (species unspecified) | LD50 | unreported | 175mg/kg (175mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 39(11), Pg. 13, 1974. | |
| mouse | LD50 | intracrebral | 90mg/kg (90mg/kg) | Pesticide Biochemistry and Physiology. Vol. 8, Pg. 302, 1978. | |
| mouse | LD50 | intraperitoneal | 75mg/kg (75mg/kg) | National Technical Information Service. Vol. AD691-490, | |
| mouse | LD50 | oral | 282mg/kg (282mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | National Technical Information Service. Vol. OTS0570918, |
| pigeon | LDLo | intramuscular | 65mg/kg (65mg/kg) | Journal of Pharmacology and Experimental Therapeutics. Vol. 49, Pg. 187, 1933. | |
| rabbit | LDLo | oral | 600mg/kg (600mg/kg) | GASTROINTESTINAL: CHANGES IN STRUCTURE OR FUNCTION OF SALIVARY GLANDS BEHAVIORAL: ATAXIA | National Technical Information Service. Vol. 0TS0518150, |
| rabbit | LDLo | skin | 1500mg/kg (1500mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: MUSCLE WEAKNESS | National Technical Information Service. Vol. 0TS0518150, |
| rat | LD50 | oral | 202mg/kg (202mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA | National Technical Information Service. Vol. 0TS0518152, |
| rat | LD50 | skin | 1024mg/kg (1024mg/kg) | National Technical Information Service. Vol. 0TS0516686, | |
| rat | LDLo | subcutaneous | 200mg/kg (200mg/kg) | BEHAVIORAL: MUSCLE WEAKNESS BEHAVIORAL: COMA | Revue Medicale de la Suisse Romande. Vol. 16, Pg. 449, 1896. |
Related Products
- 4-Nitrophenol
- 1000279-69-5
- 1000-30-2
- 1000306-34-2
- 1000335-21-6
- 1000335-27-2
- 1000339-10-5
- 1000339-23-0
- 1000339-30-9
- 1000339-51-4
- 1000340-18-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View