-
Name
5-Fluorotryptamine
- EINECS
- CAS No. 576-16-9
- Article Data21
- CAS DataBase
- Density 1.25 g/cm3
- Solubility
- Melting Point 273-275 °C
- Formula C10H11FN2
- Boiling Point 345.959 °C at 760 mmHg
- Molecular Weight 178.209
- Flash Point 163.03 °C
- Transport Information
- Appearance
- Safety 26-36
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Indole,3-(2-aminoethyl)-5-fluoro- (6CI,7CI,8CI);2-(5-Fluoro-1H-indol-3-yl)ethylamine;2-(5-Fluoroindol-3-yl)ethylamine;
- PSA 41.81000
- LogP 2.50850
Synthetic route
-
-
208645-53-8
5-fluoro-3-(2-nitrovinyl)indole

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; for 40h; | 92% |
| With lithium aluminium tetrahydride In tetrahydrofuran Reflux; |
-
-
214417-26-2
(Ε)-5-fluoro-3-(2-nitroethenyl)-1Η-indole

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 0 - 20℃; Inert atmosphere; | 76% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 5h; Ambient temperature; | |
| With lithium aluminium tetrahydride In tetrahydrofuran at 20℃; for 5h; |

| Conditions | Yield |
|---|---|
| With pyridoxal 5'-phosphate; aromatic L-amino acid decarboxylase In various solvent(s) at 30℃; for 48h; | 41% |
| With NH4OH-NH4Cl buffer; pyridoxal 5'-phosphate at 30℃; for 0.5h; relative rate of CO2 evolution by aromatic L-amino acid decarboxylase from Micrococcus percitreus; |

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| Stage #1: 5-fluoro-3-(2-nitroethyl)-1Η-indole With ammonium chloride In tetrahydrofuran; ethanol at 85℃; for 0.166667h; Stage #2: With iron | 26.2% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride |
-
-
6346-09-4
4-aminobutyrylaldehyde diethylacetal

-
-
371-14-2
4-fluorophenylhydrazine

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| With zinc(II) chloride at 180℃; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: POCl3 / 0.25 h / 20 °C 1.2: CaCO3 / 1,2-dichloro-ethane / 0.5 h / Heating 2.1: 60 percent / ammonium acetate / 1.5 h / Heating 3.1: LiAlH4 / tetrahydrofuran / 5 h / 20 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 1.) POCl3, 2.) CaCO3 / 1.) RT, 15 min, 2.) 1,2-dichloroethane, reflux, 30 min 2: 60 percent / ammonium acetate / 1.5 h / Heating 3: LiAlH4 / tetrahydrofuran / 5 h / Ambient temperature View Scheme | |
| Multi-step reaction with 3 steps 1.1: trichlorophosphate / 1.25 h / 0 - 38 °C 1.2: 0.08 h / 170 °C 2.1: ammonium acetate / 1.5 h / Reflux 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 40 h / 0 - 20 °C View Scheme | |
| Multi-step reaction with 4 steps 1: diethyl ether / 3 h / 0 - 20 °C 2: diethyl ether / 1 h / 20 °C 3: ammonia / water / 24 h / 20 °C 4: lithium aluminium tetrahydride / tetrahydrofuran / 10 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / ammonium acetate / 1.5 h / Heating 2: LiAlH4 / tetrahydrofuran / 5 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 60 percent / ammonium acetate / 1.5 h / Heating 2: LiAlH4 / tetrahydrofuran / 5 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: piperidine; acetic acid / benzene / Reflux 2: lithium aluminium tetrahydride / tetrahydrofuran / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: ammonium acetate / 1.5 h / Reflux 2: lithium aluminium tetrahydride / tetrahydrofuran / 40 h / 0 - 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: aqueous HCl; acetic acid 2: aq.-ethanolic KOH 3: aqueous HCl View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq.-ethanolic KOH 2: aqueous HCl View Scheme |
-
-
937256-55-8
3-(2-nitroethyl)-6-fluoroindole

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| With hydrazine; nickel In ethanol at 20℃; for 1h; |
-
-
16686-11-6
2-(3-chloropropyl)-1,3-dioxolane

-
-
371-14-2
4-fluorophenylhydrazine

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| In ethanol; water at 95℃; for 1h; |

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| Stage #1: C15H19FN2O2 With trifluoroacetic acid In dichloromethane at 25℃; Inert atmosphere; Stage #2: With sodium hydroxide In dichloromethane; water at 25℃; pH=Ca. 12 - Ca. 13; Inert atmosphere; | 12.6 mg |
| With trifluoroacetic acid In dichloromethane at 0 - 25℃; Inert atmosphere; | 12.6 mg |
-
-
1198292-69-1
C18H13FN2O2

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| With hydrazine In ethanol at 20℃; Combinatorial reaction / High throughput screening (HTS); |
-
-
1258297-02-7
3-(2-azidoethyl)-5-fluoro-1H-indole

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethanol under 3102.97 Torr; |
-
-
54322-20-2
4-chloro-1-hydroxy-1-butanesulfonate sodium

-
-
823-85-8
4-fluorophenyhydrazine hydrochloride

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| With disodium hydrogenphosphate In ethanol at 70℃; for 6h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium nitrite; hydrogenchloride / water / 1 h / 0 - 5 °C 1.2: 4 h / 80 - 100 °C 2.1: disodium hydrogenphosphate / ethanol / 6 h / 70 °C View Scheme |

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran for 10h; Reflux; |
-
-
408356-39-8
methyl 2-(5-fluoro-1H-indol-3-yl)-2-oxoacetate

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ammonia / water / 24 h / 20 °C 2: lithium aluminium tetrahydride / tetrahydrofuran / 10 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: diethyl ether / 1 h / 20 °C 2: ammonia / water / 24 h / 20 °C 3: lithium aluminium tetrahydride / tetrahydrofuran / 10 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 24h; Heating / reflux; | 100% |
-
-
576-16-9
5-fluorotryptamine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
1059175-54-0
tert-butyl N-[2-(5-fluoro-1H-indol-3-yl)ethyl]carbamate

| Conditions | Yield |
|---|---|
| In 1,4-dioxane at 20℃; for 16h; | 99% |
| With triethylamine In tetrahydrofuran at 10℃; for 1h; Inert atmosphere; | 83% |
| With triethylamine In tetrahydrofuran at 10℃; for 1h; | 83% |
-
-
50-00-0
formaldehyd

-
-
576-16-9
5-fluorotryptamine

-
-
22120-36-1
2-(5-fluoro-1H-indol-3-yl)-N,N-dimethylethanamine

| Conditions | Yield |
|---|---|
| With sodium acetate; sodium cyanoborohydride In methanol at 0 - 25℃; for 5h; | 97.3% |
| With sodium cyanoborohydride; acetic acid In methanol for 2h; pH=6.5; Reflux; |

| Conditions | Yield |
|---|---|
| Stage #1: 5-fluorotryptamine; salicylaldehyde In ethanol for 1h; Stage #2: With sodium cyanoborohydride In ethanol | 92.5% |
-
-
576-16-9
5-fluorotryptamine

-
-
1146222-77-6
7-(trifluoromethyl)-3,4,5,6-tetrahydro-2H-azepine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In dichloromethane at 20℃; for 168h; Pictet-Spengler Synthesis; | 90% |
-
-
576-16-9
5-fluorotryptamine

-
-
13130-10-4
5-(4-hydroxyphenyl)-2-furancarboxaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 5-fluorotryptamine; 5-(4-hydroxyphenyl)-2-furancarboxaldehyde In methanol at 20℃; Molecular sieve; Stage #2: With sodium tetrahydroborate In methanol for 1h; Molecular sieve; | 82% |
-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane for 0.5h; Heating; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-methylsalicylic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In acetone at 20℃; for 4h; Stage #2: 5-fluorotryptamine In acetone at 20℃; for 24h; | 79.43% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-hydroxy-p-toluic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In acetone at 20℃; for 4h; Stage #2: 5-fluorotryptamine In acetone at 20℃; for 24h; | 79.43% |

| Conditions | Yield |
|---|---|
| Stage #1: salicylic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In acetone at 20℃; for 4h; Stage #2: 5-fluorotryptamine In acetone at 20℃; for 24h; | 78.23% |
-
-
576-16-9
5-fluorotryptamine

-
-
24424-99-5
di-tert-butyl dicarbonate

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 0 - 20℃; | 77% |
| With dmap In dichloromethane at 0 - 20℃; for 1h; | 46% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-chlorosalicylic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In acetone at 20℃; for 4h; Stage #2: 5-fluorotryptamine In acetone at 20℃; for 24h; | 75.54% |
-
-
576-16-9
5-fluorotryptamine

-
-
1178541-58-6
(11bS)-8-fluoro-11b-phenyl-1,2,5,6,11,11b-hexahydro-3H-indolizino[8,7-b]indol-3-one

| Conditions | Yield |
|---|---|
| With (R)-3,3'-bis(triphenylsilyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate In toluene at 110℃; for 24h; optical yield given as %ee; enantioselective reaction; | 73% |
-
-
576-16-9
5-fluorotryptamine

-
-
1146222-76-5
6-(trifluoromethyl)-2,3,4,5-tetrahydropyridine

| Conditions | Yield |
|---|---|
| With camphor-10-sulfonic acid In water at 130℃; for 14h; Pictet-Spengler Synthesis; | 73% |
-
-
576-16-9
5-fluorotryptamine

-
-
32315-10-9
bis(trichloromethyl) carbonate

-
-
24036-91-7
19-nor-Δ1,3,5(10)-22α-spirostatriene-3-ol

| Conditions | Yield |
|---|---|
| Stage #1: bis(trichloromethyl) carbonate; 19-nor-Δ1,3,5(10)-22α-spirostatriene-3-ol With N-ethyl-N,N-diisopropylamine In dichloromethane at 0℃; for 0.5h; Inert atmosphere; Stage #2: 5-fluorotryptamine In dichloromethane at 20℃; for 3h; Inert atmosphere; | 73% |


| Conditions | Yield |
|---|---|
| Stage #1: 5-bromosalicyclic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In acetone at 20℃; for 4h; Stage #2: 5-fluorotryptamine In acetone at 20℃; for 24h; | 71.54% |
-
-
576-16-9
5-fluorotryptamine

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
1024493-18-2
N-(2-(5-fluoro-1H-indol-3-yl)ethyl)-4-methylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 24℃; for 4h; | 71% |
| With triethylamine In dichloromethane at 0 - 20℃; for 6h; | |
| With triethylamine In dichloromethane at 0℃; | |
| With triethylamine In dichloromethane at 20℃; for 4h; |

| Conditions | Yield |
|---|---|
| Stage #1: 5-chloro-2-hydroxybenzoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In acetone at 20℃; for 4h; Stage #2: 5-fluorotryptamine In acetone at 20℃; for 24h; | 70.54% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 26℃; for 2h; | 68.2% |
-
-
576-16-9
5-fluorotryptamine

-
-
7560-50-1, 71093-79-3, 58045-41-3
methyl (2E)-3-(4-formylphenyl)acrylate

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In tetrahydrofuran | 67% |
-
-
576-16-9
5-fluorotryptamine

-
-
339029-35-5
1-(3-chloro-5-(trifluoromethyl)pyrid-2-yl)-4-piperidone

| Conditions | Yield |
|---|---|
| Stage #1: 5-fluorotryptamine; 1-(3-chloro-5-(trifluoromethyl)pyrid-2-yl)-4-piperidone In chloroform for 1h; Molecular sieve; Heating / reflux; Stage #2: With hydrogenchloride; water In chloroform Heating / reflux; Stage #3: With sodium hydrogencarbonate In chloroform; water | 67% |
-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 25℃; for 3h; | 66% |
-
-
576-16-9
5-fluorotryptamine

-
-
26822-37-7
N,N-dimethyl-4-oxopiperidinium iodide

-
-
151191-79-6
1-[2-(5-fluoro-1H-indol-3-yl)-ethyl]-piperidin-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol | 65% |
-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| With camphor-10-sulfonic acid In water at 130℃; for 14h; Pictet-Spengler Synthesis; | 63% |

-
-
576-16-9
5-fluorotryptamine

-
-
1452838-28-6
N-[2-(5-fluoro-1H-indol-3-yl)ethyl]but-3-yne-1-sulfonamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -78℃; Inert atmosphere; | 57% |
-
-
695212-95-4
(4-dimethylamino-4-phenyl-cyclohexylmethyl)-carbamic acid phenyl ester

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 24h; Heating / reflux; | 56% |
| In 1,4-dioxane for 24h; Heating / reflux; |
-
-
55474-90-3
(4,6-dimethylpyrimidin-2-yl)cyanamide

-
-
576-16-9
5-fluorotryptamine

| Conditions | Yield |
|---|---|
| In 1,4-dioxane Heating; | 53% |
-
-
27550-90-9
2-methylmorpholine

-
-
576-16-9
5-fluorotryptamine

-
-
4457-32-3
4-nitrobenzyl chloroformate

-
-
163885-14-1
N-(p-Nitrobenzyloxycarbonyl)-5-fluorotryptamine

| Conditions | Yield |
|---|---|
| In dichloromethane | 53% |

5-FLUOROTRYPTAMINE Chemical Properties
CAS: 576-16-9
Formula: C10H11FN2
Structure of 5-Fluorotryptamine(576-16-9 ):
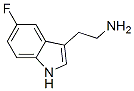
Molecular weight: 178.21
Boiling point: 346 °C at 760 mmHg
Density: 1.249 g/cm3
Flash point: 163 °C
Index of refraction: 1.643
Molar refractivity: 51.6 cm3
Molar volume: 142.5 cm3
Polarizability: 20.45 10-24cm3
Surface tension: 52.3 dyne/cm
Enthalpy of vaporization: 59.01 kJ/mol
Vapour pressure: 5.95E-05 mmHg at 25°C
Water solubility: 3.2e+004(mg/L) at 25 °C
The chemical synonyms of 5-Fluorotryptamine( 576-16-9 ) are 5-FLUOROTRYPTAMINE;AKOS B030092;3-(2-AMINOETHYL)-5-FLUORO-1H-INDOLE;2-(5-FLUORO-1H-INDOL-3-YL)-ETHYLAMINE;RARECHEM AN KA 1417;3-(2-Aminoethyl)-5-fluoro-1H-indole hydrochloride;5-FLUOROTRYPTAMINE,HPLC 98%.
The classification of 5-Fluorotryptamine ( 576-16-9 ) are: Indole/indoline/oxindole;Indole and Indoline;Indoline & Oxindole;Indole.
5-FLUOROTRYPTAMINE Safety Profile
Hazard Codes
Xi:
Xn:

Risk statements about 5-Fluorotryptamine .
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed .
R36/37/38: Irritating to eyes, respiratory system and skin .
Safety statements about 5-Fluorotryptamine .
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36: Wear suitable protective clot.
Related Products
- 5-FLUOROTRYPTAMINE
- 5-Fluorotryptamine hydrochloride
- 57618-26-5
- 576-19-2
- 576-22-7
- 576-23-8
- 576-24-9
- 57-62-5
- 5762-56-1
- 57625-74-8
- 57625-97-5
- 576-26-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View