-
Name
7-Azaindole
- EINECS 205-981-0
- CAS No. 271-63-6
- Article Data65
- CAS DataBase
- Density 1.242 g/cm3
- Solubility
- Melting Point 105-107 °C(lit.)
- Formula C7H6N2
- Boiling Point 273.8 °C at 760 mmHg
- Molecular Weight 118.138
- Flash Point 124.8 °C
- Transport Information
- Appearance brown-cyrstalline solid
- Safety 39-26-36/37/39
- Risk Codes 36-41-37/38-22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms 1,7-Dideazapurine;7H-Pyrrolo(2,3-b)pyridine;7-Aza-1-pyrindine;2,9-diazabicyclo[4.3.0]nona-2,4,7,10-tetraene;7H-Pyrrolo[2, 3-b]pyridine;7-Azaindole 98%;7-Azaindole,1H-Pyrrolo[2,3-b]pyridine;
- PSA 28.68000
- LogP 1.56290
Synthetic route
-
-
138343-77-8
1-(tert-butoxycarbonyl)-1H-pyrrolo<2,3-b>pyridine

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,2-dimethoxyethane; water for 20h; Heating; | 100% |
| With water at 100℃; for 1h; | 99% |
| With potassium phosphate In methanol at 120℃; under 5171.62 Torr; for 0.0333333h; Microwave irradiation; | 98% |
-
-
138343-75-6
tert-butyl (3-methylpyridin-2-yl)carbamate

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (3-methylpyridin-2-yl)carbamate With n-butyllithium; N,N-dimethyl-formamide In tetrahydrofuran at 0℃; Stage #2: With hydrogenchloride In tetrahydrofuran; water at 50℃; | 100% |
| With n-butyllithium In tetrahydrofuran; hexane; N,N-dimethyl-formamide at -15 - 25℃; for 17.5h; | 3.8 g |
| Stage #1: tert-butyl (3-methylpyridin-2-yl)carbamate With trimethylsilyl bromide In tetrahydrofuran; N,N-dimethyl-formamide at -30℃; for 1.75h; Inert atmosphere; Stage #2: With hydrogenchloride In tetrahydrofuran; water; N,N-dimethyl-formamide at 0 - 40℃; for 3h; | |
| Multi-step reaction with 2 steps 1.1: n-butyllithium / tetrahydrofuran / 4 h / 0 °C 1.2: 0 °C 2.1: hydrogenchloride / water / 8 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: methyl bromide; tert-butyl (3-methylpyridin-2-yl)carbamate In diethyl ether at 0℃; for 4h; Stage #2: With N,N-dimethyl-formamide In diethyl ether at 0℃; for 0.5h; Concentration; | 99.1% |
-
-
10592-27-5
7-azaindoline

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With carbon dioxide; DBN; Eosin Y In dimethyl sulfoxide at 25 - 30℃; for 48h; Irradiation; | 97% |
| With potassium tert-butylate In decane at 150℃; for 36h; Inert atmosphere; Schlenk technique; | 90% |
| With 4-tert-Butylcatechol; oxygen In water at 20℃; for 20h; Green chemistry; | 90% |
-
-
146336-83-6
(Z)-2-amino-3-(2-ethoxyethenyl)pyridine

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 2h; Heating; | 94% |
-
-
65326-33-2
1-(2-amino-3-pyridinyl)-1-ethanone

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol; water at 90℃; pH=3; Temperature; pH-value; Microwave irradiation; | 93.2% |
-
-
143141-23-5
1-benzenesulfonyl-1H-pyrrolo[2,3-b]pyridine

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In 1,4-dioxane at 80℃; for 3h; Inert atmosphere; | 93% |
-
-
136818-50-3
7-azaindole-2-carboxylic acid

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With zinc(II) oxide In toluene at 90℃; for 2h; Temperature; | 91.8% |
-
-
55052-24-9
1H-Pyrrolo[2,3-b]pyridine-7-oxide

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With (4,4′-di-tert-butyl-2,2′-bipyridine)bis[(2-pyridinyl)phenyl]iridium(III) hexafluorophosphate; di-tert-butyl 1,4-dihydro-2,6-dimethyl-3,5-pyridine-dicarboxylate In acetonitrile at 20℃; for 2h; Inert atmosphere; Irradiation; chemoselective reaction; | 91% |
| With diethyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate In dichloromethane at 31℃; for 16h; Sealed tube; Irradiation; | 83% |
| With styrene; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; hydrazine hydrate In dimethyl sulfoxide at 20℃; for 13h; Inert atmosphere; Irradiation; chemoselective reaction; | 74% |
-
-
348640-02-8
1-[(4-methylphenyl)sulfonyl]-1H-pyrrolo[2,3-b]pyridine

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With cetyltrimethylammonim bromide; potassium hydroxide In tetrahydrofuran; water for 10h; Reagent/catalyst; Temperature; Time; Reflux; Green chemistry; | 90% |
| With potassium tert-butylate In dimethyl sulfoxide at 20℃; for 1h; Inert atmosphere; Darkness; Schlenk technique; | 87% |
| With formic acid; (4,4'-di-tert-butyl-2,2'-dipyridyl)-bis-(2-phenylpyridine(-1H))-iridium(III) hexafluorophosphate; N-ethyl-N,N-diisopropylamine In acetonitrile at 20℃; for 24h; Inert atmosphere; Sealed tube; Irradiation; | 70% |
| With caesium carbonate In tetrahydrofuran; methanol at 22℃; for 2h; |

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With silver hexafluoroantimonate; bis[dichloro(pentamethylcyclopentadienyl)iridium(III)] In benzene at 130℃; for 24h; Sealed tube; Glovebox; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water for 48h; Reflux; Large scale; | 72.9% |
-
-
866319-00-8
5-fluoro-1H-pyrrolo[2,3‐b]pyridine

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With 2-methyl-propan-1-ol; methanesulfonato(2-dicyclohexylphosphino -2’,6’-di-i-propoxy-1,1‘-biphenyl)(2’-amino-1,1’-biphenyl-2-yl)palladium(II); sodium tert-pentoxide In toluene at 80℃; for 48h; Sealed tube; Inert atmosphere; | 68% |
-
-
138343-75-6
tert-butyl (3-methylpyridin-2-yl)carbamate

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (3-methylpyridin-2-yl)carbamate With n-butyllithium In tetrahydrofuran at -10 - 0℃; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran at 0 - 15℃; Stage #3: With hydrogenchloride; water at -5 - 5℃; | 67% |

| Conditions | Yield |
|---|---|
| With lithium diisopropyl amide In tetrahydrofuran at -20℃; for 5h; Reagent/catalyst; Inert atmosphere; | 65% |
-
-
933768-06-0
2-phenyloxepino[2,3-b]pyridine

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With acetamide; potassium tert-butylate In dimethyl sulfoxide at 80℃; for 48h; Inert atmosphere; | 52% |

-
A
-
271-63-6
7-Azaindole

-
B
-
1239864-85-7
C10H14N2Si

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In 1,4-dioxane at 80℃; for 3h; Inert atmosphere; | A 44% B 42% |
-
-
844503-09-9
3-ethenyl-2-nitropyridine

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 1,10-Phenanthroline; carbon monoxide In acetonitrile at 120℃; under 4560.31 Torr; for 180h; | 41% |
-
-
111097-45-1
1-acetyl-2,3-dihydropyrrolo<2,3-b>pyridine

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With n-Dodecylamine In 1,2-dichloro-ethane for 1h; Irradiation; | 25% |

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran; toluene at 90℃; for 0.5h; | 25% |

| Conditions | Yield |
|---|---|
| With perchloric acid adsorbed on silica gel; anthranilic acid amide In acetonitrile at 80℃; for 12h; | 25% |

| Conditions | Yield |
|---|---|
| With bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate; XPhos In tert-butyl alcohol at 110℃; for 24h; Sealed tube; Inert atmosphere; | 9% |

| Conditions | Yield |
|---|---|
| With sodium ethanolate at 350℃; | |
| With sodium formate; sodium anilide at 290 - 310℃; | |
| With hydrogenchloride In diethyl ether; water; pentane at -20℃; Inert atmosphere; Reflux; |
-
-
99066-81-6
1H-pyrrolo[2,3-b]pyridine-3,6-dicarboxylic acid

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| at 320 - 330℃; |
-
-
75-21-8
oxirane

-
-
74420-15-8
3-bromo-1H-pyrrolo[2,3-b]pyridine

-
A
-
271-63-6
7-Azaindole

-
B
-
90929-77-4
1-(2-hydroxyethyl)-7-azaindole

-
C
-
90929-76-3
3-(2-hydroxyethyl)-7-azaindole

| Conditions | Yield |
|---|---|
| With n-butyllithium 1.) ether, 1,2-dimethoxyethane, -5 deg C, 1 h; 2.) 0 deg C, 5 h; Yield given. Multistep reaction. Yields of byproduct given; |


-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With hydrogenchloride for 8h; Heating; Yield given; |
-
-
53277-42-2
1-(1H-pyrrolo[2,3-b]pyridin-1-yl)ethanone

-
A
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 89 percent / H2 / Pd-C / ethanol; 1,2-dimethoxy-ethane / 5 h / 20 °C 2: 25 percent / dodecylamine / 1,2-dichloro-ethane / 1 h / Irradiation View Scheme |
-
-
86847-66-7
2,2-dimethyl-N-(3-methylpyridin-2-yl)propionamide

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 1.) n-BuLi / 1.) THF, -5 to 0 deg C, 4 h, 2.) THF, 0 deg C, 40 min 2: 5.5 M HCl / 8 h / Heating View Scheme |
-
-
15128-82-2
3-hydroxy-2-nitropyridine

-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 52 percent / 60percent NaH / benzene / 60 h / Heating 2: 74 percent / Pd(PPh3)2Cl2, Et4NCl / acetonitrile / 1 h / Heating 3: 78 percent / H2 / W-2 Raney Ni / methanol 4: 94 percent / conc. HCl / methanol / 2 h / Heating View Scheme |
-
-
271-63-6
7-Azaindole

-
-
79-04-9
chloroacetyl chloride

-
-
83393-47-9
2-chloro-1-(1H-pyrrolo[2,3-b]pyridin-3-yl)ethan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: 7-Azaindole With aluminum (III) chloride In dichloromethane at 20℃; for 1.5h; Inert atmosphere; Stage #2: chloroacetyl chloride In dichloromethane at 0 - 20℃; for 18h; Inert atmosphere; | 100% |
| With aluminium trichloride In carbon disulfide at 50℃; for 2h; | 82% |
| With aluminium trichloride In carbon disulfide at 50℃; for 2h; Product distribution; | 81% |

| Conditions | Yield |
|---|---|
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide at 0℃; for 1h; Inert atmosphere; Stage #2: methyl iodide In N,N-dimethyl-formamide for 1h; Inert atmosphere; | 100% |
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide at 0℃; for 1h; Inert atmosphere; Stage #2: methyl iodide In N,N-dimethyl-formamide for 1h; Inert atmosphere; | 100% |
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide at 0℃; for 1h; Inert atmosphere; Stage #2: methyl iodide In N,N-dimethyl-formamide at 0 - 20℃; Inert atmosphere; | 98% |
-
-
271-63-6
7-Azaindole

-
-
76513-69-4
(2-trimethylethylsilylethoxy)methyl chloride

-
-
879132-46-4
1-((2-(trimethylsilyl)ethoxy)methyl)-1H-pyrrolo[2,3-b]pyridine

| Conditions | Yield |
|---|---|
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 1.25h; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In N,N-dimethyl-formamide; mineral oil for 1.25h; | 100% |
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 1.25h; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In N,N-dimethyl-formamide; mineral oil for 1.25h; | 100% |
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0℃; for 1h; Stage #2: (2-trimethylethylsilylethoxy)methyl chloride In N,N-dimethyl-formamide; mineral oil for 1h; | 100% |
-
-
271-63-6
7-Azaindole

-
-
89898-96-4
7-nitroquinoxaline-2(1H)-one

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In N,N-dimethyl-formamide at 80℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: n-pentyl halide In N,N-dimethyl-formamide at 20℃; for 15h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; trans-1,2-Diaminocyclohexane; copper(l) iodide In 1,4-dioxane at 110℃; for 24h; | 100% |
| With triethylamine In N,N-dimethyl-formamide at 110℃; for 3h; | 96% |
| With copper(l) iodide; manganese(II) fluoride; (1R,2R)-1,2-diaminocyclohexane; potassium hydroxide In water at 60℃; for 24h; | 93% |
-
-
626-55-1
3-Bromopyridine

-
-
271-63-6
7-Azaindole

-
-
847801-47-2
1-(pyridin-3-yl)-1H-pyrrolo[2,3-b]pyridine

| Conditions | Yield |
|---|---|
| With potassium phosphate; trans-1,2-Diaminocyclohexane; copper(l) iodide In 1,4-dioxane at 110℃; for 95h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: 1-Bromopentane In N,N-dimethyl-formamide at 20℃; for 3.5h; | 100% |
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: 1-Bromopentane In N,N-dimethyl-formamide at 20℃; for 3.5h; | 100% |
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: 1-Bromopentane In N,N-dimethyl-formamide at 20℃; for 3.5h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 20℃; | 100% |
-
-
271-63-6
7-Azaindole

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide In tetrachloromethane; chloroform for 4h; Ambient temperature; | 99% |
| With 1-aza-4,6,11-trioxa-5-boratricyclo[3.3.3.0(1,5)]undecane; nickel diacetate; chlorine at 90℃; for 3h; Reflux; | 99.3% |
| With 1,3-dichloro-5,5-dimethylhydantoin In 1,4-dioxane at 80℃; for 0.5h; | 92% |
| With sodium hypochlorite; sodium azide; acetic acid In water; toluene at 20℃; for 0.5h; | 91% |
| With 1-chloro-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one In N,N-dimethyl-formamide at 20℃; for 12h; regioselective reaction; | 77% |

| Conditions | Yield |
|---|---|
| With zinc diacetate In trifluoroacetic acid at 120℃; for 6h; Solvent; Concentration; | 99.1% |
-
-
271-63-6
7-Azaindole

-
-
74420-15-8
3-bromo-1H-pyrrolo[2,3-b]pyridine

| Conditions | Yield |
|---|---|
| With 1-aza-4,6,11-trioxa-5-boratricyclo[3.3.3.0(1,5)]undecane; bromine; nickel diacetate at 100℃; for 3h; Temperature; Reflux; | 99% |
| With N-Bromosuccinimide In dichloromethane at 20℃; for 16h; | 96% |
| With N-Bromosuccinimide In N,N-dimethyl-formamide at 20℃; for 4h; Cooling with ice; | 96% |
-
-
271-63-6
7-Azaindole

-
-
98-09-9
benzenesulfonyl chloride

-
-
143141-23-5
1-benzenesulfonyl-1H-pyrrolo[2,3-b]pyridine

| Conditions | Yield |
|---|---|
| Stage #1: 7-Azaindole With tetrabutylammomium bromide; sodium hydroxide In dichloromethane Stage #2: benzenesulfonyl chloride In dichloromethane at 0 - 20℃; | 99% |
| With dmap; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 98% |
| With N-benzyl-N,N,N-triethylammonium chloride; sodium hydroxide In dichloromethane at 20℃; for 2h; Cooling with ice; | 95% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; Addition; Michael addition; | 99% |
-
-
271-63-6
7-Azaindole

-
-
22445-41-6
3,5-dimethylphenyl iodide

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; (S,S)-1,2-diaminocyclohexane In 1,4-dioxane; dodecane at 110℃; for 24h; | 99% |
| With copper(I) oxide; potassium phosphate; N1,N2-bis(furan-2-ylmethyl)oxalamide In acetonitrile at 80℃; | 94% |
| With potassium phosphate monohydrate; iron(III) chloride hexahydrate; trans-N,N'-dimethylcyclohexane-1,2-diamine In water at 135℃; for 24h; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: n-butyl halide In N,N-dimethyl-formamide at 20℃; for 15h; | 99% |
-
-
271-63-6
7-Azaindole

-
-
1109-15-5
tris(pentafluorophenyl)borate

-
-
879269-87-1
7-[tris(pentafluorophenyl)borane]-7-azaindole

| Conditions | Yield |
|---|---|
| In dichloromethane (N2); a soln. of ligand added at room temp. to a soln. of B(C6F5)3, stirred for 1 h; evapd. (vac.); elem. anal.; | 99% |

| Conditions | Yield |
|---|---|
| With hafnium tetrakis(trifluoromethanesulfonate) In dichloromethane at 20℃; for 0.2h; Inert atmosphere; regioselective reaction; | 99% |
-
-
1120-90-7
3-iodopyridine

-
-
271-63-6
7-Azaindole

-
-
847801-47-2
1-(pyridin-3-yl)-1H-pyrrolo[2,3-b]pyridine

| Conditions | Yield |
|---|---|
| With manganese(II) fluoride; (S,S)-1,2-diaminocyclohexane; caesium carbonate In water at 130℃; for 24h; Reagent/catalyst; | 99% |
| With copper(l) iodide; manganese(II) fluoride; (1R,2R)-1,2-diaminocyclohexane; potassium hydroxide In water at 100℃; for 48h; | 94% |
| With copper(I) oxide; caesium carbonate In dimethyl sulfoxide at 100℃; for 24h; Reagent/catalyst; Solvent; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; Inert atmosphere; Stage #2: bromopentene In N,N-dimethyl-formamide at 0 - 20℃; Inert atmosphere; | 99% |
-
-
271-63-6
7-Azaindole

-
-
100-97-0
hexamethylenetetramine

-
-
4649-09-6
1H-pyrrolo[2,3-b]pyridin-3-carbaldehyde

| Conditions | Yield |
|---|---|
| With propionic acid In ethanol at 80℃; for 6h; Temperature; | 98.9% |
| Stage #1: 7-Azaindole; hexamethylenetetramine With acetic acid for 6h; Duff reaction; Heating; Stage #2: With water at 20℃; Further stages.; | 77% |
| With acetic acid In water for 12h; Heating / reflux; | 76% |
-
-
271-63-6
7-Azaindole

-
-
143487-47-2
cyclopentyl trimethoxysilane

| Conditions | Yield |
|---|---|
| Stage #1: 7-Azaindole With n-butyllithium In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; Cooling with ice; Stage #2: cyclopentyl trimethoxysilane In tetrahydrofuran at 0 - 20℃; for 8h; Inert atmosphere; | 98.3% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In dichloromethane at 20℃; for 2h; Friedel-Crafts Alkylation; | 98.3% |

| Conditions | Yield |
|---|---|
| In benzene for 2h; Heating; | 98% |
-
-
271-63-6
7-Azaindole

-
-
23616-57-1
3-iodo-1H-pyrrolo[2,3-b]pyridine

| Conditions | Yield |
|---|---|
| Stage #1: 7-Azaindole With potassium hydroxide In N,N-dimethyl-formamide at 20℃; for 0.166667h; Inert atmosphere; Stage #2: With iodine In N,N-dimethyl-formamide at 20℃; for 0.5h; Inert atmosphere; | 98% |
| With iodine; potassium hydroxide In N,N-dimethyl-formamide at 20℃; for 0.75h; Inert atmosphere; | 97% |
| With iodine; potassium hydroxide In N,N-dimethyl-formamide at 20℃; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: 7-Azaindole With sodium hydride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: n-propyl halide In N,N-dimethyl-formamide at 20℃; for 15h; | 98% |
-
-
271-63-6
7-Azaindole

-
-
122-60-1
Phenyl glycidyl ether

-
-
1017278-65-7
1-phenoxy-3-(1H-pyrrolo[2,3-b]pyridin-1-yl)propan-2-ol

| Conditions | Yield |
|---|---|
| ruthenium trichloride at 20℃; for 4h; Friedel-Crafts alkylation; | 98% |
| With ruthenium-exchanged FAU-Y zeolite at 20℃; for 0.5h; Friedel Crafts alkylation; Ultrasound irradiation; Neat (no solvent); regioselective reaction; | 98% |
7-Azaindole Chemical Properties
Molecule structure of 7-Azaindole (CAS NO.271-63-6):
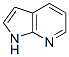
IUPAC Name: 1H-Pyrrolo[2,3-b]pyridine
Molecular Weight: 118.13594 g/mol
Molecular Formula: C7H6N2
Density: 1.242 g/cm3
Melting Point: 105-107 °C(lit.)
Boiling Point: 273.8 °C at 760 mmHg
Flash Point: 124.8 °C
Index of Refraction: 1.696
Molar Refractivity: 36.61 cm3
Molar Volume: 95 cm3
Polarizability: 14.51×10-24 cm3
Surface Tension: 60.1 dyne/cm
Enthalpy of Vaporization: 49.15 kJ/mol
Vapour Pressure: 0.0094 mmHg at 25 °C
XLogP3: 1.8
H-Bond Donor: 1
H-Bond Acceptor: 1
Tautomer Count: 2
Exact Mass: 118.053098
MonoIsotopic Mass: 118.053098
Topological Polar Surface Area: 28.7
Heavy Atom Count: 9
Canonical SMILES: C1=CC2=C(NC=C2)N=C1
InChI: InChI=1S/C7H6N2/c1-2-6-3-5-9-7(6)8-4-1/h1-5H,(H,8,9)
InChIKey: MVXVYAKCVDQRLW-UHFFFAOYSA-N
EINECS: 205-981-0
Product Categories: blocks; IndolesOxindoles; Heterocycles; Heterocycles series; Indole; Heterocyclic Compounds; Indole Series; Indole Derivatives; Pharmaceutical intermediate
7-Azaindole Uses
7-Azaindole (CAS NO.271-63-6) is used for organic synthetic reagent.
7-Azaindole Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 490mg/kg (490mg/kg) | Journal of Medicinal Chemistry. Vol. 6, Pg. 480, 1963. |
7-Azaindole Safety Profile
Hazard Codes:  Xi,
Xi,  Xn
Xn
Risk Statements: 36-41-37/38-22
R36:Irritating to eyes.
R41:Risk of serious damage to the eyes.
R37/38:Irritating to respiratory system and skin.
R22:Harmful if swallowed.
Safety Statements: 39-26-36/37/39
S39:Wear eye / face protection.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany: 3
RTECS: UY8710000
HazardClass: IRRITANT
HS Code: 29339990
7-Azaindole Specification
7-Azaindole (CAS NO.271-63-6) is also named as 1,7-Diazaindene ; 1,7-Dideazapurine ; 7-Aza-1-pyrindine ; 7H-Pyrrolo(2,3-b)pyridine ; NSC 67063 ; 1H-Pyrrolo(2,3-b)pyridine . 7-Azaindole (CAS NO.271-63-6) is brown-cyrstalline solid.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View