-
Name
8-Hydroxyquinoline
- EINECS 205-711-1
- CAS No. 148-24-3
- Article Data163
- CAS DataBase
- Density 1.26 g/cm3
- Solubility Insoluble in water
- Melting Point 70-73 °C(lit.)
- Formula C9H7NO
- Boiling Point 267 °C at 760 mmHg
- Molecular Weight 203.241
- Flash Point 143.1 °C
- Transport Information
- Appearance Cream-colored crystals
- Safety 45-36/37/39-26-36
- Risk Codes 22-68-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 8-Quinolinol;8-Chinolinol;8-Chinolinol [Czech];8-Hydroxy-chinolin [German];8-Quinol;Bioquin;CCRIS 340;Oxychinolin;Oxyquinoline;Phenopyridine;Quinoline, 8-hydroxy-;
- PSA 33.12000
- LogP 1.94040
Synthetic route

| Conditions | Yield |
|---|---|
| With RhCl(PPh3)3 In benzene at 40℃; for 1h; Product distribution; primer deut. isotop eff.; | A 100% B n/a |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In decane at 150℃; for 36h; Inert atmosphere; Schlenk technique; | 98% |
| With iron oxide surrounded by nitrogen doped graphene shell immobilized on carbon support In acetonitrile at 100℃; under 11251.1 Torr; for 24h; Autoclave; | 93% |
| With perylene diimide covalent immobilized to SiO2 nanospheres; air In N,N-dimethyl acetamide at 20℃; UV-irradiation; | 93% |
-
-
52968-25-9
Bis-<8-chinolinyl>-carbonat

-
-
81494-56-6
2-Amino-1,1,3,3-tetramethoxypropan

-
A
-
148-24-3
8-quinolinol

| Conditions | Yield |
|---|---|
| In chloroform 1.) r. t., 12 h, 2.) reflux, 10 h; | A 97% B 74% |


| Conditions | Yield |
|---|---|
| Stage #1: glycerol With sulfuric acid; copper(II) sulfate; calcium oxide for 1h; Reflux; Stage #2: 2-amino-phenol for 0.333333h; Skraup Quinoline Synthesis; Stage #3: With 2-hydroxynitrobenzene at 130 - 140℃; Concentration; | 96.31% |
| Stage #1: 2-amino-phenol With sulfuric acid; 2-hydroxynitrobenzene at 70℃; for 0.5h; Stage #2: glycerol at 150℃; for 4h; Temperature; | 91% |
| With sulfuric acid In water at 36 - 200℃; for 0.25h; Microwave irradiation; Sealed tube; Green chemistry; | 34% |
| With sulfuric acid; iodine | |
| Stage #1: 2-amino-phenol; glycerol With sulfuric acid Stage #2: With nitrobenzene |

| Conditions | Yield |
|---|---|
| Stage #1: anthranilic acid; acrolein With hydrogenchloride; acetic acid; hydroquinone at 95 - 100℃; for 3h; Stage #2: With chloropyridinecobaloxime(III); Eosin Y In water; acetonitrile at 20℃; for 3h; Microwave irradiation; | 96.2% |

| Conditions | Yield |
|---|---|
| With formic acid at 95 - 100℃; for 2h; Concentration; Molecular sieve; | 95.6% |


| Conditions | Yield |
|---|---|
| With silica gel at 110℃; for 0.25h; isomerization; | A 5% B 95% |

-
-
148-24-3
8-quinolinol

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In neat (no solvent, solid phase) at 20℃; for 0.583333h; Green chemistry; | 94% |

| Conditions | Yield |
|---|---|
| With nickel(III) oxide; sulfuric acid; acetic acid at 90℃; for 5h; Temperature; | 92.7% |
| With sulfuric acid; acetic acid |

-
-
108530-08-1
quinolin-8-yl trifluoromethanesulfonate

-
-
148-24-3
8-quinolinol

| Conditions | Yield |
|---|---|
| With caesium carbonate In toluene at 80℃; for 15h; | 92% |

| Conditions | Yield |
|---|---|
| With bis(triphenyl)oxodiphosphonium trifluoromethanesulfonate salt; potassium iodide In ethanol at 20℃; for 1.16667h; | 90% |
| With lithium tetrafluoroborate In water; acetonitrile at 20℃; for 4h; Electrochemical reaction; Inert atmosphere; | 24 mg |
-
-
222713-64-6
8-((tert-butyldimethylsilyl)oxy)quinoline

-
-
148-24-3
8-quinolinol

| Conditions | Yield |
|---|---|
| With zinc tetrafluoroborate In water for 13h; Ambient temperature; | 89% |
| With potassium hydrogen difluoride In methanol at 20℃; for 2h; | 89% |

| Conditions | Yield |
|---|---|
| With piperidine; carbon monoxide; palladium diacetate; triethylamine; triphenylphosphine In N,N-dimethyl-formamide at 50℃; under 52505.3 Torr; for 24h; | 83% |

| Conditions | Yield |
|---|---|
| With water; poly(ethyleneimine) at 40℃; for 0.5h; | 82% |

-
-
148-24-3
8-quinolinol

| Conditions | Yield |
|---|---|
| With ammonia In water at 20℃; for 24h; | 80.6% |

| Conditions | Yield |
|---|---|
| With tetrachloromethane; iron(III) chloride hexahydrate at 150℃; for 8h; Inert atmosphere; Sealed tube; | 79% |
| With 2,4,6-trimethyl-pyridine; oxygen; palladium diacetate; trifluoroacetic acid at 150℃; for 16h; Schlenk technique; | 55% |

| Conditions | Yield |
|---|---|
| With i-Amyl alcohol; ozone In water at -0.1℃; | 78% |
| With dihydrogen peroxide; bis(triphenyl)oxodiphosphonium trifluoromethanesulfonate salt In ethanol; water at 20℃; for 1h; regioselective reaction; | 55% |
| findet sich im Harn von Kaninchen nach peroraler Verabreichung; |

| Conditions | Yield |
|---|---|
| With lithium salt of proline; tetrabutylammomium bromide; potassium hydroxide; copper dichloride In water at 120℃; for 0.666667h; Microwave irradiation; Green chemistry; | 78% |
-
-
16567-18-3
8-bromoquinoline

-
-
148-24-3
8-quinolinol

| Conditions | Yield |
|---|---|
| With 5-(di-tert-butylphosphino)-1′, 3′, 5′-triphenyl-1′H-[1,4′]bipyrazole; tris-(dibenzylideneacetone)dipalladium(0); cesiumhydroxide monohydrate In 1,4-dioxane at 110℃; Inert atmosphere; Glovebox; Sealed tube; | 74% |
-
-
23504-14-5
2-(prop-2-yn-1-ylamino)phenol

-
A
-
148-24-3
8-quinolinol

-
B
-
343270-95-1
2-methylidene-3,4-dihydro-2H-1,4-benzoxazine

| Conditions | Yield |
|---|---|
| With potassium carbonate; gold(I) chloride In acetonitrile for 8h; Reflux; | A 8% B 72% |
-
-
6640-50-2
1,2,3,4-tetrahydroquinolin-8-ol

-
-
98-95-3
nitrobenzene

-
A
-
148-24-3
8-quinolinol

-
B
-
62-53-3
aniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 58% B 62% |

-
-
6640-50-2
1,2,3,4-tetrahydroquinolin-8-ol

-
-
20651-75-6
1-butyl-4-nitro-benzene

-
A
-
148-24-3
8-quinolinol

-
B
-
104-13-2
4-Butylaniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 62% B 61% |


| Conditions | Yield |
|---|---|
| With oxygen; N-ethyl-N,N-diisopropylamine; [5,6]fullerene-C70 In chloroform; toluene at 20℃; for 12h; Irradiation; | 61% |
-
-
83-73-8
5,7-diiodo-8-hydroxyquinoline

-
A
-
148-24-3
8-quinolinol

-
B
-
13207-63-1
5-iodoquinolin-8-ol

-
C
-
7385-89-9
7-iodo-8-hydroxyquinoline

| Conditions | Yield |
|---|---|
| With carbon monoxide; glycine ethyl ester hydrochloride; palladium diacetate; triethylamine; triphenylphosphine In N,N-dimethyl-formamide at 50℃; under 52505.3 Torr; for 24h; | A 51% B n/a C n/a |

-
-
6640-50-2
1,2,3,4-tetrahydroquinolin-8-ol

-
-
7369-50-8
3-ethylnitrobenzene

-
A
-
148-24-3
8-quinolinol

-
B
-
587-02-0
m-ethylaniline

| Conditions | Yield |
|---|---|
| With nickel-nitrogen-doped carbon framework In water at 145℃; for 18h; Inert atmosphere; Sealed tube; Green chemistry; | A 50% B 51% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 111℃; for 24h; Doebner-von Miller Reaction; | 41% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; silver trifluoromethanesulfonate In toluene at 80℃; for 4h; | 40% |
-
-
1245937-15-8
C19H15NO3

-
-
148-24-3
8-quinolinol

| Conditions | Yield |
|---|---|
| at 650℃; Flash vacuum pyrolysis; | 34% |
-
-
91-22-5
quinoline

-
A
-
580-18-7
3-hydroxyquinoline

-
B
-
148-24-3
8-quinolinol

-
C
-
118-92-3
anthranilic acid

-
D
-
112259-26-4
(5R,6S)-cis-5,6-dihydroxy-5,6-dihydroquinoline

| Conditions | Yield |
|---|---|
| With Pseudomonas putida | A 13% B 27% C 27% D 33% |
| biotransformation by Pseudomonas putida UV4; Further byproducts given; | A 11 mg B 23 mg C 22 mg D 32% |
| biotransformation by Pseudomonas putida UV4; Further byproducts given; | A 11 mg B 23 mg C 22 mg D 32 mg |


| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 24h; Heating; | 100% |
| With sodium hydroxide; tetrabutylammomium bromide In dichloromethane; water at 25℃; for 4h; | 84% |
| With potassium carbonate In ethanol at 70℃; for 10h; regioselective reaction; | 84% |
-
-
148-24-3
8-quinolinol

-
-
121-57-3
4-aminobenzene sulfonic acid

-
-
574-70-9
(E)-4-((8-hydroxyquinolin-5-yl)diazenyl)benzenesulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-aminobenzene sulfonic acid With hydrogenchloride; sodium nitrite In water at -5℃; for 0.25h; Stage #2: 8-quinolinol With sodium hydroxide In water | 100% |
| With hydrogenchloride; sodium nitrite | |
| With hydrogenchloride; sodium hydroxide; ethylenediaminetetraacetic acid; sodium nitrite In ethanol for 0.0833333h; |

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane | 100% |
| With dihydrogen peroxide In water at 20℃; for 4h; Catalytic behavior; | 98% |
| With 3-chloro-benzenecarboperoxoic acid In Isopropyl acetate at 10 - 35℃; for 5.83333h; Green chemistry; | 98.2% |

| Conditions | Yield |
|---|---|
| With oleum at 20℃; | 100% |
| With sulfuric acid | |
| With sulfuric acid at 180℃; im Rohr; | |
| With sulfuric acid at 0 - 5℃; for 30h; |
-
-
148-24-3
8-quinolinol

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
108530-08-1
quinolin-8-yl trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 12h; Schlenk technique; Inert atmosphere; | 100% |
| With pyridine In dichloromethane at 0 - 20℃; | 99% |
| With pyridine In dichloromethane at 0 - 20℃; | 97% |
-
-
148-24-3
8-quinolinol

-
-
85116-37-6
(-)-diisopinocamphenylborane chloride

| Conditions | Yield |
|---|---|
| In diethyl ether | 100% |
-
-
148-24-3
8-quinolinol

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; antimony pentafluoride at 25℃; | 100% |
| With trifluorormethanesulfonic acid; cyclohexane; antimony pentafluoride at 20℃; |
-
-
148-24-3
8-quinolinol

-
-
10025-73-7
chromium(III) chloride

| Conditions | Yield |
|---|---|
| With ammonia In water prepn. from aq. CrCl3 soln. and a soln. of 8-hydroxy-quinoline (in CH3CO2H, neutralized by dropwise addn. of NH4OH (slight pptn.), cleared by heating, satd. with CO2 after cooling, adjusted to pH 4-8 with aq. NH3, heated for 1.5 h with stirring;; pptn., filtering hot, washing with hot H2O and drying at 110 °C;; | 100% |
| With NH3 In water prepn. from aq. CrCl3 soln. and a soln. of 8-hydroxy-quinoline (in CH3CO2H, neutralized by dropwise addn. of NH4OH (slight pptn.), cleared by heating, satd. with CO2 after cooling, adjusted to pH 4-8 with aq. NH3, heated for 1.5 h with stirring;; pptn., filtering hot, washing with hot H2O and drying at 110 °C;; | 100% |

| Conditions | Yield |
|---|---|
| In diethyl ether To a suspn. of complex in ether was added stoichiometric amount of ligand, stirred for 1-2 h at room temp.;; recrystd. from CH2Cl2-n-hexane; elem. anal.;; | 100% |
-
-
148-24-3
8-quinolinol

| Conditions | Yield |
|---|---|
| With triethylamine In diethyl ether; benzene byproducts: (Et3NH)Cl; N2 atmosphere, addn. of Et3N and Et2O soln. of quinolinol compound to benzene soln. of Mo complex at room temp., stirring (18 h); removement of volatiles (vacuum), extn. (pentane), centrifugation, concn. of pentane layers; elem. anal.; | 100% |
-
-
148-24-3
8-quinolinol

-
-
5893-61-8, 14690-98-3, 15305-21-2, 16842-73-2, 22992-79-6, 34729-06-1, 133386-02-4, 133386-04-6
copper(II) formate tetrahydrate

-
-
13014-03-4
copper 8-hydroxyquinolinate

| Conditions | Yield |
|---|---|
| at 80 - 100℃; under 0.000750075 Torr; for 1h; Time; Large scale; | 100% |
-
-
148-24-3
8-quinolinol

-
-
150-13-0
4-amino-benzoic acid

-
-
67940-27-6
(E)-4-((8-hydroxyquinolin-5-yl)diazenyl)benzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-amino-benzoic acid With hydrogenchloride; sodium nitrite In water at -5℃; for 0.25h; Stage #2: 8-quinolinol With sodium hydroxide In water | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 8-quinolinol With sodium hydride In tetrahydrofuran Inert atmosphere; Stage #2: di-tert-butyl-diazodicarboxylate In tetrahydrofuran at -5 - 60℃; for 1.5h; Reagent/catalyst; Solvent; Time; Temperature; Inert atmosphere; | 99.9% |

| Conditions | Yield |
|---|---|
| Stage #1: 8-quinolinol With sodium hydride In tetrahydrofuran Inert atmosphere; Stage #2: di-isopropyl azodicarboxylate In tetrahydrofuran at -5 - 60℃; for 1.5h; Reagent/catalyst; Solvent; Time; Temperature; Inert atmosphere; | 99.7% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 40 - 90℃; for 1h; | 99.3% |

| Conditions | Yield |
|---|---|
| With bromine; sodium hydrogencarbonate In methanol at 20℃; for 0.0833333h; | 99% |
| With bromine In methanol at 20℃; for 0.0833333h; | 97% |
| Stage #1: 8-quinolinol With bromine; sodium hydrogencarbonate In methanol at 20℃; for 0.0833333h; Stage #2: With sodium sulfite In methanol; water at 20℃; | 97% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; 2-carbomethoxy-3-hydroxyquinoxaline-di-N-oxide; caesium carbonate In N,N-dimethyl-formamide at 110℃; for 12h; Schlenk technique; Inert atmosphere; | 99% |
| With potassium carbonate In dimethyl sulfoxide for 0.0833333h; Heating; microwave irradiation; | 94% |
| With PEG-400; sodium hydroxide In N,N-dimethyl-formamide for 15h; Etherification; Irradiation; microwave irradiation; | 54% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 0 - 20℃; for 4h; | 99% |
| With hydrogenchloride In water at 0 - 20℃; for 8h; | 98% |
| With hydrogenchloride In water at 0 - 20℃; for 8h; Product distribution / selectivity; | 98% |

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 99% |
| With triethylamine In dichloromethane at 0 - 20℃; | 94% |
| With triethylamine In ethyl acetate at 0 - 20℃; for 0.166667h; Green chemistry; | 85% |

| Conditions | Yield |
|---|---|
| In triethylamine byproducts: {(C2H5)3NH}Cl; sublimation at 200 °C and 10E-1 Torr;; | 99% |
| In triethylamine byproducts: {(C2H5)3NH}Cl; sublimation at 200 °C and 10E-1 Torr;; | 99% |
| In triethylamine byproducts: {(C2H5)3NH}Cl; | 68% |
| In triethylamine byproducts: {(C2H5)3NH}Cl; | 68% |
-
-
148-24-3
8-quinolinol

-
-
10025-69-1
tin(II) chloride dihdyrate

-
-
12148-41-3
bis(8-hydroxyquinolinate)tin(II)

| Conditions | Yield |
|---|---|
| In ethanol N2-atmosphere; pptn. on dropwise addn. of SnCl2 to ligand (room temp., stirring); washing (EtOH, water), drying (vac., 1 h); | 99% |
-
-
148-24-3
8-quinolinol

-
-
15120-50-0
pentamethylantimony

-
-
22784-64-1
tetramethyl(8-oxyquinolinato)antimony(V)

| Conditions | Yield |
|---|---|
| In toluene byproducts: CH4; refluxing Me5Sb and oxine in toluene for 24 h; evapn. of solvent in vacuo, elem. anal.; | 99% |
-
-
148-24-3
8-quinolinol

-
-
632323-52-5
nitridobis(1,3-diphenylpropane-1,3-dionate)chromium(V)

-
-
632323-51-4
nitridobis(8-hydroxyquinolinate)chromium(V)

| Conditions | Yield |
|---|---|
| In acetonitrile addn. of chromium complex to a soln. of ligand in acetonitrile, reflux for 5 min; hot filtration, cooling; elem. anal.; | 99% |
-
-
148-24-3
8-quinolinol

-
-
55171-05-6, 13814-75-0
molybdenum(VI) tetrachloride oxide

| Conditions | Yield |
|---|---|
| In tetrachloromethane MoOCl4 and equiv. amt. of 8-hydroxyquinoline in CCl4 at room temp.; | 99% |
-
-
148-24-3
8-quinolinol

-
-
55171-05-6, 13814-75-0
molybdenum(VI) tetrachloride oxide

| Conditions | Yield |
|---|---|
| In tetrachloromethane MoOCl4 and equiv. amt. of 8-hydroxyquinoline in CCl4 at room temp.; | 99% |

| Conditions | Yield |
|---|---|
| In tetrachloromethane WOCl4 and equiv. amt. of 8-hydroxyquinoline in CCl4 at room temp.; | 99% |

| Conditions | Yield |
|---|---|
| In tetrachloromethane WOCl4 and equiv. amt. of 8-hydroxyquinoline in CCl4 at room temp.; | 99% |
-
-
148-24-3
8-quinolinol

-
-
511243-86-0
5-[(6-Bicyclo[2.2.1]hept-5-en-2-yl-hexylamino)-methyl]-quinolin-8-ol

-
-
557-20-0
diethylzinc

-
-
796882-43-4
(C7H9(CH2)6NHCH2C9H5NO)zinc(8-hydroxyquinoline)

| Conditions | Yield |
|---|---|
| In not given 1:1:1 molar ratio, ligand added to ZnEt2, followed by quinoline; | 99% |


| Conditions | Yield |
|---|---|
| In chloroform for 72h; Reflux; Schlenk technique; Inert atmosphere; | 99% |
8-Hydroxyquinoline Analytical Methods
It is usually prepared from quinoline-8-sulfonic acid and from a Skraup synthesis from 2-aminophenol.
8-Hydroxyquinoline Specification
The 8-Hydroxyquinoline, also known as 8-Quinolinol, is the organic compound with the formula C9H7NO. It belongs to the product categories of Inorganic & Organic Chemicals; Fine Chemical & Intermediates; Quinolines, Quinazolines and derivatives; Quinolines; Analytical Chemistry; Bipyridyls, etc. (Chelating Reagents); Chelating Reagents; Hydroxyquinolines; Pharmaceutical intermediates; Miscellaneous Compounds; Aromatics; Miscellaneous Reagents. Its EINECS registry number is 205-711-1. With the CAS registry number 148-24-3, its IUPAC name is quinolin-8-ol. This chemical is white to off-white or faintly yellow crystalline powder with phenolic odor. What's more, it is insoluble in water.
Physical properties of 8-Hydroxyquinoline: (1)ACD/LogP: 1.901; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.77; (4)ACD/LogD (pH 7.4): 0.82; (5)ACD/BCF (pH 5.5): 1.23; (6)ACD/BCF (pH 7.4): 1.35; (7)ACD/KOC (pH 5.5): 19.25; (8)ACD/KOC (pH 7.4): 21.28; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Index of Refraction: 1.691; (13)Molar Refractivity: 44.068 cm3; (14)Molar Volume: 115.203 cm3; (15)Surface Tension: 59.73 dyne/cm; (16)Density: 1.26 g/cm3; (17)Flash Point: 143.07 °C; (18)Enthalpy of Vaporization: 52.536 kJ/mol; (19)Boiling Point: 266.999 °C at 760 mmHg
Preparation: It is usually prepared from quinoline-8-sulfonic acid and from a Skraup synthesis from 2-aminophenol. This chemical can also be prepared by quinoline. This reaction will need reagent O3, isopentyl alcohol and solvent H2O. The reaction temperature is -0.1 °C. The yield is about 78%.
Uses of 8-Hydroxyquinoline: it can be used to produce 8-butoxy-quinoline. This reaction will need reagent ethanol and KOH-solution.
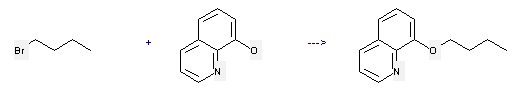
This colorless compound is widely used commercially, although under a variety of names. Solution of 8-Hydroxyquinoline in alcohol is used as liquid bandages. It once was of interest as an anti-cancer drug. The reaction of 8-Hydroxyquinoline with aluminium(III) results in Alq3, a common component of organic light-emitting diodes (OLED's). Variations in the substituents on the quinoline rings affect its luminescence properties. The roots of the invasive plant Centaurea diffusa release 8-Hydroxyquinoline, which has a negative effect on plants that have not co-evolved with it.
When you are using this chemical, please be cautious about it as the following:
This chemical may cause inflammation to the skin or other mucous membranes. It is irritating to eyes, respiratory system and skin. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)InChI: InChI=1S/C9H7NO/c11-8-5-1-3-7-4-2-6-10-9(7)8/h1-6,11H
(2)InChIKey: MCJGNVYPOGVAJF-UHFFFAOYSA-N
(3)Canonical SMILES : C1=CC2=C(C(=C1)O)N=CC=C2
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD20 | oral | 1205mg/kg (1205mg/kg) | Teratology, The International Journal of Abnormal Development. Vol. 20, Pg. 413, 1979. | |
| mammal (species unspecified) | LD50 | unreported | 1gm/kg (1000mg/kg) | "Chemistry of Pesticides," Melnikov, N.N., New York, Springer-Verlag New York, Inc., 1971Vol. -, Pg. 410, 1971. | |
| mouse | LD50 | intraperitoneal | 43mg/kg (43mg/kg) | Farmakologiya i Toksikologiya Vol. 42, Pg. 396, 1979. | |
| mouse | LD50 | oral | 20gm/kg (20000mg/kg) | Drugs in Japan Vol. 6, Pg. 271, 1982. | |
| mouse | LD50 | subcutaneous | 83600ug/kg (83.6mg/kg) | Pharmazie. Vol. 1, Pg. 150, 1946. | |
| rat | LC50 | inhalation | > 1210mg/m3/6H (1210mg/m3) | Journal of the American College of Toxicology. Vol. 11, Pg. 497, 1992. | |
| rat | LD50 | oral | 1200mg/kg (1200mg/kg) | Pesticide Chemicals Official Compendium, Association of the American Pesticide Control Officials, Inc., 1966. Vol. -, Pg. 602, 1966. |
Related Products
- 8-Hydroxyquinoline
- 8-Hydroxyquinoline aluminum salt
- 8-HYDROXYQUINOLINE BENZOATE
- 8-Hydroxyquinoline copper(II) salt
- 8-Hydroxyquinoline potassium sulfate
- 8-Hydroxyquinoline succinate
- 8-Hydroxyquinoline sulfate
- 8-Hydroxyquinoline sulfate
- 8-Hydroxyquinoline sulfate monohydrate
- 8-Hydroxyquinoline-2-carboxaldehyde
- 148244-82-0
- 148249-36-9
- 148-25-4
- 148256-63-7
- 14825-82-2
- 1482-62-8
- 14826-50-7
- 148274-47-9
- 1482-82-2
- 1482-97-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View