-
Name
Acyclovir
- EINECS 261-685-1
- CAS No. 59277-89-3
- Article Data50
- CAS DataBase
- Density 1.77 g/cm3
- Solubility Water: 0.7 mg/mL
- Melting Point 256-257 °C
- Formula C8H11N5O3
- Boiling Point 595 °C at 760 mmHg
- Molecular Weight 225.207
- Flash Point 313.6 °C
- Transport Information
- Appearance White to light yellow crystal powder
- Safety 22-24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Acyclovir
- PSA 119.05000
- LogP -0.75060
Synthetic route
-
-
75128-73-3
2-acetylamino-9-(2-acetoxyethoxymethyl)purine-6-one

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 85 - 95℃; for 3h; | 97.5% |
| With methylamine In water at 23℃; for 1h; | 93% |
| With sodium hydroxide | 92% |
-
-
131490-71-6
1-<(2-hydroxyethoxy)methyl>-5-<(thiocarbamoyl)amino>-1H-imidazole-4-carboxamide

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; copper diacetate for 1h; Heating; | 92% |
| With sodium hydroxide; copper(II) ion at 100℃; for 1h; Product distribution; further metal ions and reaction conditions investigatet; reaction also with H2O2 / Na2WO4; | 92% |
-
-
112233-78-0
9-<(2-acetoxyethoxy)methyl>-2-N-acetyl-6-O-(diphenylcarbamoyl)guanine

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol; water at 60℃; | 91% |
| With ammonium hydroxide In methanol at 60℃; for 24h; | 91% |
-
-
1336-21-6
ammonium hydroxide

-
-
75128-73-3
2-acetylamino-9-(2-acetoxyethoxymethyl)purine-6-one

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| In methanol | 90% |
-
-
81777-49-3
2-amino-6-chloro-9-<(2-hydroxyethoxy)methyl>-9H-purine

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With phosphoric acid; adenosine deaminase Ambient temperature; pH=7.5; | 89% |
-
-
59277-86-0
2,6-diamino-9-{(2-hydroxyethoxy)methyl}purine

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With phosphoric acid; adenosine deaminase Ambient temperature; pH=7.5; | 89% |
-
-
364634-35-5
9-(2-trimethylsilyloxyethoxymethyl)guanine

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With acetic acid In water at 80℃; for 2h; | 85% |

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In methanol for 24h; Ambient temperature; | 84% |
-
-
127218-19-3
Acetic acid 2-(2-bromo-6-oxo-1,6-dihydro-purin-9-ylmethoxy)-ethyl ester

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 120℃; | 80% |
-
-
1400637-35-5
2-[(2-amino-6-oxo-3,6-dihydro-9H-purin-9-yl)methoxy]ethyl pyrrolidinophosphate ammonium salt

-
A
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With potassium chloride; potassium hydroxide In water at 25℃; pH=12; | A 80% B n/a |
-
-
1400637-36-6
2-[(2-amino-6-oxo-3,6-dihydro-9H-purin-9-yl)methoxy]ethyl morpholinophosphate ammonium salt

-
A
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With potassium chloride; potassium hydroxide In water at 25℃; for 120h; pH=12; | A 80% B n/a |
-
-
1400637-37-7
N-(2-morpholinoethyl)-2-[(2-amino-6-oxo-3,6-dihydro-9H-purin-9-yl)methoxy]ethyl phosphoramidate ammonium salt

-
A
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With potassium chloride; potassium hydroxide In water at 25℃; pH=12; | A 80% B n/a |

-
A
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With potassium chloride; potassium hydroxide In water at 25℃; pH=12; | A 80% B n/a |
-
-
1400637-39-9
9-(2-hydroxyethoxymethyl)guanine phosphoromono-N,N-dimethylaminoethylamidate

-
A
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With potassium chloride; potassium hydroxide In water at 25℃; pH=12; | A 80% B n/a |
-
-
1400637-40-2
9-(2-hydroxyethoxymethyl)guanine phosphoromono-3-dimethylamino-1-propylamidate

-
A
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With potassium chloride; potassium hydroxide In water at 25℃; pH=12; | A 80% B n/a |
-
-
1400637-42-4
N-hexyl-2-[(2-amino-6-oxo-3,6-dihydro-9H-purin-9-yl)methoxy]ethyl phosphoramidate ammonium salt

-
A
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With potassium chloride; potassium hydroxide In water at 25℃; pH=12; | A 80% B n/a |
-
-
646-06-0
1,3-DIOXOLANE

-
-
73-40-5
2-amino-1,9-dihydro-6H-purin-6-one

-
-
7440-44-0
pyrographite

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; acetic acid; 1,1,1,3,3,3-hexamethyl-disilazane | 78% |
| With trifluorormethanesulfonic acid; 1,1,1,3,3,3-hexamethyl-disilazane In water; acetic acid | 73% |
| With ammonium hydroxide; sodium hydroxide; trifluorormethanesulfonic acid; acetic acid; 1,1,1,3,3,3-hexamethyl-disilazane In water; toluene | 72% |
| With trifluorormethanesulfonic acid; acetic acid; 1,1,1,3,3,3-hexamethyl-disilazane In methanol; water | 66.6% |


-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With ammonia In methanol | 75% |

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With acetic acid In water | 69% |
-
-
661465-32-3
acyclovir P-(O-isopropyl) hydrogenphosphonate

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With human blood serum In water at 37℃; for 72h; Product distribution; Further Variations:; Reagents; | 52% |
-
-
59277-92-8
1-(2-amino-6-chloro-purin-9-ylmethoxy)-2-benzoyloxy-ethane

-
-
60-24-2
2-hydroxyethanethiol

-
A
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| In methanol; methanolic sodium methylate; water | A n/a B 45% |

-
-
75128-77-7
9-<(2-hydroxyethoxy)methyl>-2-(methylthio)hypoxanthine

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With ammonia In ethanol at 140℃; for 60h; | 39% |
| In ethanol; ammonia | 15 mg (39%) |
-
-
157722-20-8
acyclovir hydrogenphosphonate

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With human blood serum In water at 37℃; for 72h; Product distribution; Further Variations:; Reagents; | 18% |
-
-
59278-00-1
2-acetoxyethyl acetoxymethyl ether

-
-
108-24-7
acetic anhydride

-
-
118-00-3
G

-
A
-
59277-89-3
acycloguanosine

-
B
-
91702-61-3
7-(2-Hydroxyethoxymethyl)guanine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; toluene-4-sulfonic acid 1.) 100 deg C, 20 h, 2.) room temperature, overnight; Yield given. Multistep reaction; | A n/a B 0.5% |

-
-
58305-05-8
(2-benzoyloxyethyl)oxymethyl chloride

-
-
73-40-5
2-amino-1,9-dihydro-6H-purin-6-one

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With ammonium sulfate; tetrabutyl ammonium fluoride 1.) hexamethyldisilazane, reflux, 24 h; 2.) THF/benzene, reflux, 3 h; Yield given. Multistep reaction; |
-
-
73-40-5
2-amino-1,9-dihydro-6H-purin-6-one

-
-
1462-33-5
2-chloroethyl chloromethyl ether

-
A
-
59277-89-3
acycloguanosine

-
B
-
127302-85-6
9-<<2-chloroethoxy>methyl>guanine

| Conditions | Yield |
|---|---|
| With ammonium sulfate; tetrabutyl ammonium fluoride 1.) hexamethyldisilazane, reflux, 24 h; 2.) THF/benzene, reflux, 3 h; Yield given. Multistep reaction. Yields of byproduct given; | |
| With ammonium sulfate; tetrabutyl ammonium fluoride 1.) hexamethyldisilazane, reflux, 24 h; 2.) THF/benzene, reflux, 4 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
73-40-5
2-amino-1,9-dihydro-6H-purin-6-one

-
-
1462-35-7
1-bromo-2-(chloromethoxy)ethane

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With ammonium sulfate; tetrabutyl ammonium fluoride 1.) hexamethyldisilazane, reflux, 24 h; 2.) THF/benzene, reflux, 3 h; Yield given. Multistep reaction; |
-
-
102728-64-3
9-[(acetoxyethoxy)methyl]guanine

-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With methylamine at 80℃; for 0.5h; | |
| With Dulbecco's Modified Phosphite-Buffer Saline; SIRC cell lysate at 34℃; pH=7.4; Kinetics; Further Variations:; Reagents; |
-
-
59277-89-3
acycloguanosine

-
-
108-24-7
acetic anhydride

-
-
110104-37-5
2-acetylamino-9-<(2-hydroxyethoxy)methyl>-1,9-dihydro-6H-purin-6-one

| Conditions | Yield |
|---|---|
| With dmap for 120h; Ambient temperature; | 98% |
-
-
59277-89-3
acycloguanosine

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

| Conditions | Yield |
|---|---|
| With N,N-dimethyl-formamide Condensation; | 98% |
-
-
59277-89-3
acycloguanosine


| Conditions | Yield |
|---|---|
| With dmap; triethylamine; bromo-tris(1-pyrrolidinyl)phosphonium hexafluorophosphate In N,N-dimethyl-formamide at 20℃; for 72h; | 98% |
-
-
59277-89-3
acycloguanosine

-
-
1036914-97-2
2-(2,2-dimethyl-3-trityloxypropanoyl)sulfanylethoxyphosphinic acid

-
-
1037071-44-5
9-(2-hydroxy-ethoxymethyl)-guanin-5'-yl-0-(triphenylmethyloxy-tert-butyl-5-acyl-2-thioethyl) H-phosphonate

| Conditions | Yield |
|---|---|
| With pivaloyl chloride In pyridine at -15 - 20℃; for 2h; | 98% |
-
-
59277-89-3
acycloguanosine

-
-
2082-76-0
capric anhydride

-
-
1360928-42-2
2-amino-9-[(2-decanoyloxy)ethyl-oxy-methyl]-1,9-dihydro-6H-purin-6-one

| Conditions | Yield |
|---|---|
| With dmap In dimethyl sulfoxide for 0.166667h; Inert atmosphere; | 98% |
-
-
59277-89-3
acycloguanosine

-
-
626-29-9
myristic anhydride

-
-
866215-87-4
2-amino-9-[(2-tetradecanoyloxy)ethyloxy-methyl]-1,9-dihydro-6H-purin-6-one

| Conditions | Yield |
|---|---|
| With dmap In dimethyl sulfoxide for 0.166667h; Inert atmosphere; | 98% |
-
-
59277-89-3
acycloguanosine

-
-
18162-48-6
tert-butyldimethylsilyl chloride

| Conditions | Yield |
|---|---|
| With 1H-imidazole at 25℃; for 48h; | 97% |
-
-
59277-89-3
acycloguanosine

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
139767-68-3
2-amino-9-[2-(tert-butyldimethylsilyloxy)ethoxymethyl]-1,9-dihydropurin-6-one

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 48h; | 97% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; | 87% |
| With 1H-imidazole In N,N-dimethyl-formamide at 23℃; for 5h; | 79% |
-
-
59277-89-3
acycloguanosine

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
100699-59-0
N'-(9-((2-hydroxyethoxy)-methyl)-6-oxo-6,9-dihydro-1H-purin-2-yl)-N,N-dimethylformimidamide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 24h; | 97% |
| In N,N-dimethyl-formamide at 20℃; |
-
-
59277-89-3
acycloguanosine

-
-
2051-49-2
n-hexanoic anhydride

| Conditions | Yield |
|---|---|
| With dmap In dimethyl sulfoxide for 0.166667h; Inert atmosphere; | 97% |
| dmap In N,N-dimethyldecanoamide at 25℃; for 48h; Product distribution / selectivity; Inert atmosphere; | 90% |
-
-
59277-89-3
acycloguanosine

-
-
645-66-9
lauric anhydride

-
-
140900-50-1
2-amino-9-[(2-dodecanoyloxy)ethyl-oxymethyl]-1,9-dihydro-6H-purin-6-one

| Conditions | Yield |
|---|---|
| With dmap In dimethyl sulfoxide for 0.166667h; Inert atmosphere; | 97% |
-
-
106-31-0
butanoic acid anhydride

-
-
59277-89-3
acycloguanosine

-
-
64843-83-0
2-amino-9-[(2-butanoyloxy)ethyl-oxy-methyl]-1,9-dihydro-6H-purin-6-one

| Conditions | Yield |
|---|---|
| With dmap In dimethyl sulfoxide for 0.166667h; Inert atmosphere; | 96% |
| With pyridine; dmap In N,N-dimethyl-formamide for 36h; |
-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| Stage #1: C20H33N3O9S With dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 0℃; for 1h; Inert atmosphere; Stage #2: acycloguanosine With dmap In N,N-dimethyl-formamide at 0 - 20℃; for 24h; Inert atmosphere; | 96% |
-
-
59277-89-3
acycloguanosine

-
-
81475-44-7
2-amino-8-bromo-9-[(2-hydroxyethoxy)methyl]-1,9-dihydro-6H-purin-6-one

| Conditions | Yield |
|---|---|
| With bromine In water at 20℃; | 95% |
| With bromine In water at 20℃; for 0.5h; | 87% |
| With bromine In water |

| Conditions | Yield |
|---|---|
| With dmap In dimethyl sulfoxide for 0.166667h; Inert atmosphere; | 95% |
| With dmap In DMF (N,N-dimethyl-formamide) at 20℃; for 18h; | 91% |
| With pyridine at 40℃; for 2h; | 78% |
| With pyridine; dmap In N,N-dimethyl-formamide for 36h; | |
| With dmap |
-
-
59277-89-3
acycloguanosine

-
-
13416-90-5
divinyl butanedioate

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase acrylic resin In acetone at 50℃; for 12h; | 95% |
-
-
59277-89-3
acycloguanosine

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
100699-59-0
N'-(9-((2-hydroxyethoxy)-methyl)-6-oxo-6,9-dihydro-1H-purin-2-yl)-N,N-dimethylformimidamide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 96h; | 95% |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 60℃; for 12h; | 94.5% |
-
-
59277-89-3
acycloguanosine

-
-
88950-64-5
1-<(tert-butyloxy)carbonylamino>cyclopropane-1-carboxylic acid

-
-
915725-88-1
1-tert-butoxycarbonylamino-cyclopropanecarboxylic acid 2-(2-amino-6-oxo-1,6-dihydro-purin-9-ylmethoxy)-ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; triethylamine In N,N-dimethyl-formamide at 20℃; | 94% |
| With dmap; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; triethylamine In N,N-dimethyl-formamide at 20℃; | 93.7% |
-
-
59277-89-3
acycloguanosine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In isopropyl alcohol at 20℃; for 0.5h; | 94% |
-
-
59277-89-3
acycloguanosine

-
-
55592-85-3
DL-N,N-di-CBZ-lysine

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 20℃; for 24h; | 91% |
-
-
59277-89-3
acycloguanosine

-
-
73-40-5
2-amino-1,9-dihydro-6H-purin-6-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water | 90% |
-
-
59277-89-3
acycloguanosine

-
-
31233-28-0
diiodo(2,9-dimethyl-1,10-phenanthroline-κ(2)N,N')platinum(II)

-
-
75-09-2
dichloromethane

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane soln. PtI2(Me2phen) (CH2Cl2) treated with soln of acyclovir (CH3OH); stirring for one day; solvent evapd. vac.; diethyl ether added; ppt. filtered; washed (ether);dried vac.; elem. anal.; | 90% |

-
-
59277-89-3
acycloguanosine

-
-
31233-28-0
diiodo(2,9-dimethyl-1,10-phenanthroline-κ(2)N,N')platinum(II)

-
-
75-09-2
dichloromethane

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane soln. PtI2(Me2phen) (CH2Cl2/CH3OH 1:1) treated with acyclovir; stirring for 2 h; solvent evapd. vac.; suspended (H2O); stirred over night; soln. filtered; evapd. vac.; elem. anal.; | 90% |

Acyclovir Specification
1. Introduction of Acyclovir
Acyclovir is one kind of white to light yellow crystal powder. IUPAC Name of IUPAC Name is 2-Amino-9-(2-hydroxyethoxymethyl)-3H-purin-6-one. Product Category of Acyclovir is Active Pharmaceutical Ingredients;Purine;Nucleotides and Nucleosides;Antivirals for Research and Experimental Use;Biochemistry;Chemical Reagents for Pharmacology Research;Nucleosides, Nucleotides & Related Reagents. It can be soluble in water which is 0.7 mg/mL.
2. Properties of Acyclovir
Physical properties about Acyclovir are:
(1)Melting point: 256-257 °C; (2)Storage tempreture: -20 °C; (3)Water solubility: 0.7 mg/mL; (4)Index of Refraction: 1.762 ; (5)Density: 1.77 g/cm3 ; (6)Flash Point: 313.6 °C ; (7)Enthalpy of Vaporization: 93.25 kJ/mol ; (8)Boiling Point: 595 °C at 760 mmHg ; (9)Vapour Pressure: 5.29E-15 mmHg at 25 °C.
3. Structure Descriptors of Acyclovir
(1)InChI: InChI=1S/C8H11N5O3/c9-8-11-6-5(7(15)12-8)10-3-13(6)4-16-2-1-14/h3,14H,1-2,4H2,(H3,9,11,12,15);
(2)InChIKey: InChIKey=MKUXAQIIEYXACX-UHFFFAOYSA-N;
(3)Smiles: c1nc2c(=O)[nH]c(nc2n1COCCO)N.
4. Toxicity of Acyclovir
Aciclovir may cause nephrotoxicity (crystallization of aciclovir within renal tubules, elevation of serum creatinine, transient), and neurotoxicity (coma, hallucinations, lethargy, seizures, tremors). Nephrotoxicity and neurotoxicity usually resolve after cessation of aciclovir therapy. However, there is no well-defined relationship between aciclovir concentrations in the blood and these adverse effects.
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | oral | 3gm/kg/22W-I (3000mg/kg) | BLOOD: AGRANULOCYTOSIS BLOOD: THROMBOCYTOPENIA | American Journal of Emergency Medicine. Vol. 16, Pg. 396, 1998. |
| mammal (species unspecified) | LD50 | intravenous | 400mg/kg (400mg/kg) | Pharmacy International. Vol. 5, Pg. 191, 1984. | |
| man | TDLo | intravenous | 134ug/kg/1D-I (0.134mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" | Lancet. Vol. 2, Pg. 385, 1985. |
| man | TDLo | intravenous | 107mg/kg/4D-I (107mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" | American Journal of Kidney Diseases. Vol. 22, Pg. 611, 1993. |
| man | TDLo | oral | 486mg/kg/17D- (486mg/kg) | GASTROINTESTINAL: DECREASED MOTILITY OR CONSTIPATION GASTROINTESTINAL: OTHER CHANGES | American Journal of Gastroenterology. Vol. 88, Pg. 2110, 1993. |
| mouse | LD50 | intraperitoneal | 724mg/kg (724mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 25, Pg. 815, 1994. | |
| mouse | LD50 | intravenous | 1118mg/kg (1118mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 16, Pg. 866, 1985. | |
| mouse | LD50 | oral | > 10gm/kg (10000mg/kg) | Nature. Vol. 272, Pg. 583, 1978. | |
| mouse | LD50 | subcutaneous | 1118mg/kg (1118mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 16, Pg. 866, 1985. | |
| rat | LD50 | intraperitoneal | 860mg/kg (860mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 16, Pg. 866, 1985. | |
| rat | LD50 | intravenous | 750mg/kg (750mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 25, Pg. 815, 1994. | |
| rat | LD50 | oral | > 20gm/kg (20000mg/kg) | Drugs in Japan Vol. -, Pg. 7, 1990. | |
| rat | LD50 | subcutaneous | 620mg/kg (620mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 16, Pg. 866, 1985. | |
| women | LDLo | multiple routes | 36mg/kg/2D-I (36mg/kg) | BRAIN AND COVERINGS: OTHER DEGENERATIVE CHANGES BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | American Journal of Kidney Diseases. Vol. 20, Pg. 647, 1992. |
| women | TDLo | intravenous | 101mg/kg/2D-I (101mg/kg) | BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) | American Journal of Medicine. Vol. 94, Pg. 212, 1993. |
| women | TDLo | oral | 12mg/kg/1D-I (12mg/kg) | BRAIN AND COVERINGS: MENINGEAL CHANGES BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ANTIPSYCHOTIC | Southern Medical Journal. Vol. 87, Pg. 1227, 1994. |
| women | TDLo | oral | 28mg/kg/2D-I (28mg/kg) | BRAIN AND COVERINGS: CHANGES IN SURFACE EEG BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" LUNGS, THORAX, OR RESPIRATION: SPUTUM | Southern Medical Journal. Vol. 87, Pg. 1227, 1994. |
| women | TDLo | oral | 80mg/kg/4D-I (80mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: "HALLUCINATIONS, DISTORTED PERCEPTIONS" | Annals of Internal Medicine. Vol. 111, Pg. 187, 1989. |
| women | TDLo | oral | 100mg/kg/5D-I (100mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, ALLERGIC: AFTER SYSTEMIC EXPOSURE" | British Medical Journal. Vol. 289, Pg. 1424, 1984. |
5. Physical Properties of Acyclovir
| Physical Property | Value | Units | Temp (deg C) | Source |
|---|---|---|---|---|
| Melting Point | 255 | deg C | EXP | |
| log P (octanol-water) | -1.56E+00 | (none) | EXP | |
| Water Solubility | 1620 | mg/L | 22 | EXP |
| Vapor Pressure | 7.47E-15 | mm Hg | 25 | EST |
| Henry's Law Constant | 3.18E-22 | atm-m3/mole | 25 | EST |
| Atmospheric OH Rate Constant | 7.94E-11 | cm3/molecule-sec | 25 | EST |
6. Safety information of Acyclovir
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 22-24/25-36-26
S22: Do not breathe dust.
S24/25: Avoid contact with skin and eyes.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36: Wear suitable protective clothing.
7. Uses of Acyclovir
Acyclovir is a commonly-used antiviral drug and is used for the treatment of herpes simplex virus infections. It is commonly marketed as tablets (200 mg, 400 mg, 800 mg and 1 gram), topical cream (5%), intravenous injection (25 mg/mL) and ophthalmic ointment (3%). It can be used especially against herpes.
Aciclovir (INN) or acyclovir is a synthetic deoxyguanosine analog and it is the prototype antiviral agent that is activated by viral thymidine kinase. The selective activity of aciclovir is due to its affinity for the thymidine kinase enzyme encoded by HSV and VZV.
8. Production of Acyclovir
(1)After Guanine trimethylsilyl and then react with 2-benzyloxyethoxy methyl chloride. Then remove the benzyl group to give acyclovir. The yield is 24%. The detailed steps are as follows:
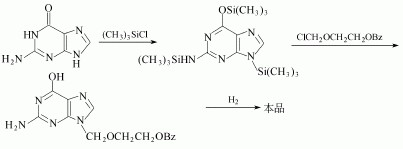
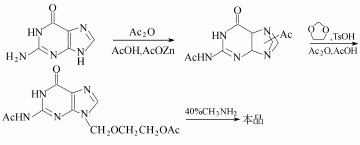
(2)N, N'-diacetyl guanine and 2-oxa-butanediol diacetate in dimethyl sulfoxide can be used to manufacture the Acyclovir.
Related Products
- Acyclovir
- 59278-00-1
- 59278-65-8
- 59279-58-2
- 59279-60-6
- 59280-70-5
- 592-82-5
- 5928-26-7
- 592-84-7
- 5928-48-3
- 5928-51-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View