-
Name
Amoxicillin
- EINECS 248-003-8
- CAS No. 26787-78-0
- Article Data30
- CAS DataBase
- Density 1.54 g/cm3
- Solubility 4g/L at 25℃
- Melting Point 140 °C
- Formula C16H19N3O5S
- Boiling Point 743.2 °C at 760 mmHg
- Molecular Weight 365.41
- Flash Point 403.3 °C
- Transport Information
- Appearance solid
- Safety 22-36/37
- Risk Codes 42/43
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms 4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylicacid, 6-[2-amino-2-(p-hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-, D- (8CI);Eupensol;Excillin;Farconcil;Fisamox;Foxolin;Fullcilina;Gemox;Gimalxina;Gomcillin;Gramidil;Grunamox;Hiconcil;Hipen;Histocillin;Hosboral;Imacillin;Imaxilin;Imox;Imoxil;Intermox;Jerramcil;4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid,6-[[amino(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-, [2S-[2a,5a,6b(S*)]]-;6-[D-(-)-p-Hydroxy-a-aminobenzyl]penicillin;6-[D-a-Amino-a-(4-hydroxyphenyl)acetamido]penicillanicacid;A-Gram;Actimoxi;Agerpen;Alfamox;Almodan;Alphamox;Amagesen Solutab;Amodex;Amoflux;Amopenixin;Amoxa;Amoxal;Amoxapen;Amoxaren;Amoxi;Amoxi-Mast;Amoxicilina;Amoxidal;Amoxiden;Amoxil;Amoxin;Amoxipen;Amoxtrex;Amoxy;Amoxypen;Ampidroxyl;Ampy-Penyl;Anemol;Anemolin;Apitart;Apo-Amoxi;Ardine;Aspenil;Audumic;BLP1410;BRL 2333;Bactox;Betamox;Bimox;Biomox;Bioxidona;Bioxyllin;Cabermox;Cilamox;Clamox;Clamoxyl;Coamoxin;Comoxyl;D-(-)-a-Amino-p-hydroxybenzyl penicillin;D-2-Amino-2-(4-hydroxyphenyl)acetamidopenicillanic acid;D-Amoxicillin;Delacillin;Efpenix;Efpinex;Eupen;
- PSA 158.26000
- LogP 1.05280
Synthetic route

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol; water for 3h; | 62% |
-
-
551-16-6
6-Aminopenicillanic Acid

-
-
37763-23-8
D-2-p-hydroxyphenylglycine methyl ester

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| at 30℃; for 3h; Pseudomonas melanogenum KY 3987 and KY 8540; Yield given; | |
| With penicillin acylasefrom Escherichia coli; water |
-
-
551-16-6
6-Aminopenicillanic Acid

-
-
57591-61-4, 68697-60-9, 127369-30-6, 134694-94-3
methyl (R)-2-amino-2-(4-hydroxyphenyl)acetate hydrochloride

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With iso-butanol In water at 20℃; Xanthomonas citri K24, pH = 6-7; Yield given; |

| Conditions | Yield |
|---|---|
| In sodium amoxycillin; water; amoxycillin trihydrate; cefaclor monohydrate |

-
-
7732-18-5
water

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide In acetone |
-
-
32462-30-9
(S)-hydroxyphenylglycine

-
-
3282-30-2
pivaloyl chloride

-
-
141-97-9
ethyl acetoacetate

-
-
551-16-6, 1079-37-4, 3115-55-7, 21794-93-4, 145920-51-0
6-aminopenicillanic acid

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol; dichloromethane |


-
-
82095-48-5, 95510-67-1
D(-)-p-hydroxy-N-(1-ethoxycarbonyl-2-propenyl)-alpha-aminophenylacetic acid potassium salt

-
-
3282-30-2
pivaloyl chloride

-
-
551-16-6, 1079-37-4, 3115-55-7, 21794-93-4, 145920-51-0
6-aminopenicillanic acid

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| In dichloromethane; ISOPROPYLAMIDE; water |

-
-
551-16-6, 1079-37-4, 3115-55-7, 21794-93-4, 145920-51-0
6-aminopenicillanic acid

-
-
26787-78-0
amoxicillin
-
-
551-16-6, 1079-37-4, 3115-55-7, 21794-93-4, 145920-51-0
6-aminopenicillanic acid

-
A
-
26787-78-0
amoxicillin

-
B
-
760-67-8
2-ethylhexanoic acid chloride

-
-
551-16-6, 1079-37-4, 3115-55-7, 21794-93-4, 145920-51-0
6-aminopenicillanic acid

-
-
10416-58-7
N,N-bis(trimethylsilyl)acetamide

-
-
26787-78-0
amoxicillin

-
-
82095-48-5, 95510-67-1
D(-)-p-hydroxy-N-(1-ethoxycarbonyl-2-propenyl)-alpha-aminophenylacetic acid potassium salt

-
-
68698-88-4
N-triisopropylsilyl-2-oxazolidinone

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With triethylamine hydrochloride In N-methyl-acetamide; dichloromethane |
-
-
551-16-6, 1079-37-4, 3115-55-7, 21794-93-4, 145920-51-0
6-aminopenicillanic acid

-
-
822-39-9
2-chloro-1,3,2-dioxaphospholan

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; diisopropylamine In dichloromethane |

-
-
79-22-1
methyl chloroformate

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane; triethylamine In dichloromethane; N,N-dimethyl-formamide |
-
-
73590-58-6
omeprazole

-
-
26787-78-0
amoxicillin

-
-
26787-78-0
amoxicillin
-
-
732216-08-9
famotidine

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With penicillin acylasefrom Escherichia coli; water |
-
-
551-16-6
6-Aminopenicillanic Acid

-
-
43189-12-4
(R)-methyl 2-amino-2-(4-hydroxyphenyl)acetate

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With penicillin G acylase at 20℃; Enzymatic reaction; |

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol; water for 3h; |
-
-
22818-40-2
D-4-hydroxyphenylglycine

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hydrogencarbonate / tetrahydrofuran; water / 8.42 h 2.1: chloroformic acid ethyl ester; N-ethyl-N,N-diisopropylamine / 1-methyl-pyrrolidin-2-one / 0.33 h / 0 °C 2.2: -60 - 20 °C 3.1: palladium 10% on activated carbon; hydrogen / water; methanol / 3 h View Scheme |

| Conditions | Yield |
|---|---|
| With 1,4-dithio-L-threitol In water at 20℃; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 0 - 20℃; for 3h; pH=4; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 0 - 20℃; for 3h; pH=4; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In 1,4-dioxane; water at 0 - 20℃; | 97% |
| With potassium carbonate In 1,4-dioxane; water at 0 - 25℃; for 1.5h; |
-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| Stage #1: amoxicillin With ammonium hydroxide for 0.333333h; Stage #2: With sodium hydroxide at 20℃; for 4h; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: amoxicillin With ammonium hydroxide In methanol Stage #2: cholin hydroxide In methanol at 20℃; for 1h; | 93% |
-
-
951163-09-0
trihexyl(tetradecyl) phosphonium hydroxide

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| Stage #1: amoxicillin With ammonium hydroxide In methanol Stage #2: trihexyl(tetradecyl) phosphonium hydroxide In methanol at 20℃; for 1h; | 92% |
-
-
26787-78-0
amoxicillin

-
-
34642-77-8
Acuotricina

| Conditions | Yield |
|---|---|
| Stage #1: amoxicillin With triethylamine In methanol; acetic acid methyl ester at 0 - 5℃; Stage #2: With sodium 2-ethylhexanoic acid In methanol; acetic acid methyl ester at 0 - 5℃; Product distribution / selectivity; | 90% |
| With sodium hydroxide In water Product distribution / selectivity; | |
| With sodium hydrogencarbonate In water Product distribution / selectivity; | |
| With sodium carbonate In water Product distribution / selectivity; |

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| Stage #1: amoxicillin With trimethylsilyl iodide; triethylamine In dichloromethane at 0℃; for 3h; Stage #2: C5H9N2O3(1-)*K(1+) With 4-methyl-morpholine; chloroformic acid ethyl ester In dichloromethane at -10 - -5℃; for 15h; | 90% |
-
-
26787-78-0
amoxicillin

-
-
105-45-3
acetoacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; for 6h; | 86.98% |

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| In ethanol; water at -10 - -5℃; for 2.5h; pH=7.5; | 85.2% |
-
-
26787-78-0
amoxicillin


| Conditions | Yield |
|---|---|
| Stage #1: [(2,3-bis-methoxycarbonyloxy-benzoyl)-(8-{(2,3-bis-methoxycarbonyloxy-benzoyl)-[8-(8-methoxycarbonyloxy-2,4-dioxo-4H-benzo[e][1,3]oxazin-3-yl)-octyl]-amino}-octyl)-amino]-acetic acid With 4-methyl-morpholine; methyl chloroformate In tetrahydrofuran at -10℃; for 1h; Stage #2: amoxicillin With triethylamine In tetrahydrofuran | 83% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water for 2h; pH=7 - 8; Reflux; | 81% |
| In ethanol for 3h; Inert atmosphere; | 70% |

| Conditions | Yield |
|---|---|
| With 1-hydroxy-pyrrolidine-2,5-dione; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 0 - 20℃; for 3h; Inert atmosphere; | 81% |
-
-
26787-78-0
amoxicillin

-
-
477947-79-8
C11H16ClNO4Si

| Conditions | Yield |
|---|---|
| Stage #1: amoxicillin With chloro-trimethyl-silane; triethylamine In acetonitrile at 25℃; for 1h; Stage #2: C11H16ClNO4Si In acetonitrile at 25℃; for 6h; | 80% |

-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran; water; ethyl acetate; Petroleum ether | 80% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 0 - 20℃; for 3h; pH=4; Inert atmosphere; | 80% |
-
-
26787-78-0
amoxicillin

-
-
77997-18-3
1-hydro-5-(p-aminosulfonyl)anilino-2-hydroxy-oxazolo<5,4-d>pyrimidine

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran; water for 1h; | 78% |
-
-
26787-78-0
amoxicillin

-
-
501-53-1
benzyl chloroformate

| Conditions | Yield |
|---|---|
| Stage #1: amoxicillin With sodium hydroxide In tetrahydrofuran; water at 20℃; for 0.166667h; Stage #2: benzyl chloroformate In tetrahydrofuran; water Time; Cooling with ice; | 77% |

| Conditions | Yield |
|---|---|
| Stage #1: amoxicillin With ammonium hydroxide Stage #2: 1-ethyl-3-methylimidazolium hydroxide In methanol at 20℃; for 1h; | 77% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 0.0416667h; pH=7 - 8; Microwave irradiation; | 73% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 3h; Reflux; | 70% |
-
-
26787-78-0
amoxicillin

| Conditions | Yield |
|---|---|
| With sodium hydroxide; phosphate buffer In 1,4-dioxane at 0℃; for 1h; | 69% |
-
-
26787-78-0
amoxicillin

-
-
473988-39-5
3-methyl-4-oxo-imidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxylic acid N-hydroxysuccinimide ester

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20 - 30℃; for 4h; | 69% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water for 3h; Reflux; | 68% |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Reflux; Inert atmosphere; | 67% |

| Conditions | Yield |
|---|---|
| With triethylamine In water; acetone at -12 - 20℃; for 3.75h; | 66% |
-
-
26787-78-0
amoxicillin

-
-
473988-44-2
3-(2-chloroethyl)-4-oxo-imidazo[5,1-d]-1,2,3,5-tetrazine-8-carboxylic acid N-hydroxysuccinimide ester

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 20 - 30℃; for 4h; | 65% |
-
-
26787-78-0
amoxicillin

-
-
90-99-3
diphenylchloromethane

-
-
477947-68-5
diphenylmethyl 6β-[(R)-2-(diphenylmethylamino)-2-(4-hydroxyphenyl)acetamido]penicillanate

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 25℃; for 3h; | 65% |

| Conditions | Yield |
|---|---|
| With triethylamine In water; acetone at -12 - 20℃; for 3.75h; | 64% |
Amoxicillin Chemical Properties
Structure of Amoxicillin (CAS NO.26787-78-0):
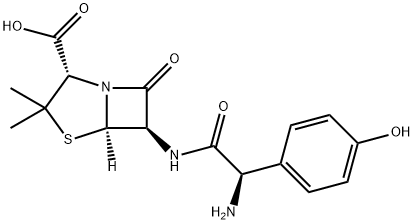
IUPAC Name: (2S,5R,6R)-6-[[(2R)-2-amino-2-(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
Empirical Formula: C16H19N3O5S
Molecular Weight: 365.4042
EINECS: 248-003-8
Index of Refraction: 1.701
Molar Refractivity: 91.47 cm3
Molar Volume: 236.2 cm3
Polarizability: 36.26×10-24cm3
Surface Tension: 85.3 dyne/cm
Density: 1.54 g/cm3
Flash Point: 403.3 °C
Enthalpy of Vaporization: 113.73 kJ/mol
Boiling Point: 743.2 °C at 760 mmHg
Vapour Pressure: 3.39E-23 mmHg at 25°C
Stability: Stable. Incompatible with strong oxidizing agents.
Product Categories: Antibiotics;A - KAntibiotics;Antibacterial;Antibiotics A to;Antibiotics A-FAntibiotics;Chemical Structure Class;Interferes with Cell Wall SynthesisAntibiotics;Mechanism of Action;Penicillins and Cephalosporins (beta-Lactams);Spectrum of Activity
Amoxicillin Uses
Amoxicillin can be used as broad-spectrum semi-synthetic PENICILLIN. It is active against gram-positive and -negative bacteri. Clinic for the treatment of tonsillitis, laryngitis, pneumonia, chronic bronchitis, urinary tract infections, skin and soft tissue infections, septic pleurisy, hepatobiliary system infections, sepsis, typhoid, dysentery. The antimicrobial spectrum of goods for drugs, and its role and application of both PENICILLIN and Benzyl chloride similar strengths of the serum protein binding rate and blood concentration more than twice as high as ampicillin.
Amoxicillin Production
6-APA and N-(3-ethoxycarbonyl-1-methyl vinyl) Sodium p-hydroxyphenylglycine derived by condensation. 6- APA is the production of a wide range of semi-synthetic PENICILLIN important raw materials. Large industrial use of microorganisms PENICILLIN lactamase cracker natural PENICILLIN (PENICILLIN G or PENICILLIN V) approach to the admission system.
Amoxicillin Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | oral | 300mg/kg (300mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" KIDNEY, URETER, AND BLADDER: HEMATURIA KIDNEY, URETER, AND BLADDER: OTHER CHANGES IN URINE COMPOSITION | Clinical Pediatrics Vol. 32, Pg. 735, 1993. |
| child | TDLo | oral | 682mg/kg (682mg/kg) | KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" KIDNEY, URETER, AND BLADDER: URINE VOLUME DECREASED KIDNEY, URETER, AND BLADDER: HEMATURIA | Clinical Pediatrics Vol. 32, Pg. 735, 1993. |
| man | TDLo | oral | 7143ug/kg (7.143mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | Allergy. Vol. 52(Suppl, |
| man | TDLo | oral | 40mg/kg/4D (40mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" GASTROINTESTINAL: OTHER CHANGES | Lancet. Vol. 2, Pg. 707, 1978. |
| mouse | LD50 | intracrebral | > 500mg/kg (500mg/kg) | BRAIN AND COVERINGS: RECORDINGS FROM SPECIFIC AREAS OF CNS | Chemotherapy Vol. 26, Pg. 196, 1980. |
| mouse | LD50 | intraperitoneal | 3590mg/kg (3590mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. | |
| mouse | LD50 | oral | > 25gm/kg (25000mg/kg) | Drugs in Japan Vol. 6, Pg. 38, 1982. | |
| mouse | LD50 | subcutaneous | > 20mg/kg (20mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. | |
| rat | LD50 | intraperitoneal | 2870mg/kg (2870mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. | |
| rat | LD50 | oral | > 15gm/kg (15000mg/kg) | Drugs in Japan Vol. 6, Pg. 38, 1982. | |
| rat | LD50 | subcutaneous | > 8gm/kg (8000mg/kg) | Drugs in Japan Vol. -, Pg. 59, 1990. |
Amoxicillin Safety Profile
Hazard Codes :  Xn,
Xn, Xi
Xi
Risk Statements : 42/43
R42/43:May cause sensitization by inhalation and skin contact.
Safety Statements : 22-36/37
S22:Do not breathe dust.
S36/37:Wear suitable protective clothing and gloves.
WGK Germany : 2
RTECS : XH8300000
Amoxicillin Standards and Recommendations
Purity of amoxicillin: 98.00%
Amoxicillin Specification
Amoxicillin , its cas register number is 26787-78-0. It also can be called 6-(D-(-)-alpha-Amino-p-hydroxyphenylacetamido)penicillanic acid ; 6-(D-(-)-p-Hydroxy-alpha-aminobenzyl)penicillin ; 6-(p-Hydroxy-alpha-aminophenylacetamido)penicillanic acid ; Amoclen ; Amolin ; Amopenixin ; Amoxi ; Amoxi-Mast ;Amoxicaps ; Amoxicilina ; Amoxicillin pediatric ; Amoxicilline ; Amoxicillinum Amoxiden ; Amoxil ; Amoxivet ; D-(-)-alpha-Amino-p-hydroxybenzylpenicillin ; D-2-Amino-2-(4-hydroxyphenyl)acetamidopenicillanic acid ; D-Amoxicillin ; Delacillin ; DisperMox ; Hiconcil ; Histocillin ; Ibiamox ; Imacillin ; Larotid ; Metafarma capsules ; Metifarma capsules ; Moxacin ; Moxal ; Wymox ; alpha-Amino-p-hydroxybenzylpenicillin ; p-Hydroxyampicillin .
Related Products
- Amoxicillin
- Amoxicillin sodium
- Amoxicillin trihydrate
- 267880-81-9
- 267880-87-5
- 267881-10-7
- 2679-14-3
- 26791-73-1
- 26791-93-5
- 26794-61-6
- 2679-65-4
- 26798-33-4
- 26798-52-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View