-
Name
Benzyl mercaptan
- EINECS 202-862-5
- CAS No. 100-53-8
- Article Data246
- CAS DataBase
- Density 1.058 g/cm3
- Solubility Not miscible or difficult to mix in water.
- Melting Point -29 °C
- Formula C7H8S
- Boiling Point 195.3 °C at 760 mmHg
- Molecular Weight 124.207
- Flash Point 70 °C
- Transport Information 2810
- Appearance colourless liquid with a leek or garlic-like odour
- Safety 23-26-36/37/39-45-61-60
- Risk Codes 22-23-50/53
-
Molecular Structure
-
Hazard Symbols
 T,
T, N
N
- Synonyms alpha-Toluolthiol;alpha-Toluenethiol;toluene-α-thiol;Benzylmercaptan;Benzyl mercaptan 96%;r-Toluenethiol;Toluene-alpha-thiol;alpha-Tolyl mercaptan;Benzyl hydrosulfide;Benzylthiol;.alpha.-Mercaptotoluene;alpha-Mercaptotoluene;.alpha.-Toluenethiol;Toluene, alpha-mercapto-;(Mercaptomethyl)benzene;Benzylhydrosulfide;benzenemethanethiol;Thiobenzyl alcohol;phenylmethanethiol;Methanethiol, phenyl-;
- PSA 38.80000
- LogP 2.11640
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrosulfide exchange resin In acetonitrile at 25℃; for 0.25h; | 98% |
| With thiourea In ethanol for 0.166667h; Reflux; | 95% |
| With hydrosulfide exchange resin (from Amberlite IRA-400); triethylamine hydrochloride In methanol for 1h; Ambient temperature; | 87% |

| Conditions | Yield |
|---|---|
| With sodium hydrogen telluride In ethanol 1.) 0 deg C, 2.) 40 deg C, 30 min; | 97% |
| With sodium hydrogen telluride In ethanol Product distribution; 1.) 0 deg C, 2.) 40 deg C, 30 min; reductive cleavage of sulfur-sulfur bonds in dialkyl, diaryl disulfides and organic thiosulfites; | 97% |
| With triethylphosphine In tetrahydrofuran; water for 1h; Ambient temperature; | 96% |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether at 22 - 25℃; for 0.5h; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: benzyl bromide With potassium carbonate; tiolacetic acid In methanol at 20℃; for 0.5h; Stage #2: With potassium carbonate In methanol at 20℃; for 0.5h; | 96% |
| With hydrosulfide exchange resin (from Amberlite IRA-400); triethylamine hydrochloride In methanol for 1h; Ambient temperature; | 88% |
| Stage #1: benzyl bromide With thiourea In water at 100℃; for 2h; Inert atmosphere; Stage #2: With sodium hydroxide In water; toluene at 60℃; for 1h; | 84% |
-
-
32362-99-5
benzylthioacetate

-
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With acetyl chloride In methanol at 25 - 30℃; for 3h; | 96% |
| With potassium carbonate In methanol at 20℃; for 0.5h; | 96% |
| With palladium diacetate In methanol for 1h; Heating; | 95% |
-
-
167872-03-9
Thiocarbonic acid S-benzyl ester O-[1-(3,5-dimethoxy-phenyl)-2-oxo-2-phenyl-ethyl] ester

-
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| In benzene for 1h; Irradiation; | 95% |
-
-
607731-44-2
1-[[(Z)-2-(benzylsulfanyl)vinyl]sulfonyl]-4-methylbenzene

-
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With pyrrolidine In acetonitrile at 20℃; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogen In water at 180℃; under 7500.75 Torr; for 5h; Reagent/catalyst; Temperature; Autoclave; | A 95% B 12% |

| Conditions | Yield |
|---|---|
| In water at 70℃; for 2h; | A 94.27% B n/a |
| at 70℃; for 2h; |

-
-
89647-16-5
1-(2-Benzylsulfanyl-2-methyl-propane-1-sulfonyl)-4-methyl-benzene

-
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tert-butyl alcohol for 0.5h; Product distribution; Ambient temperature; further reagents: CH3ONa, CH3OLi, KOH, other solvent: CH3OH; cleavage of various adducts; | 93% |
-
-
84495-76-1
N-Phenyl-thioformimidic acid benzyl ester

-
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water Ambient temperature; | 92% |

| Conditions | Yield |
|---|---|
| With magnesium; acetic acid In methanol at 20℃; | 92% |

-
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| Stage #1: C22H18O3S With sodium hypochlorite In dichloromethane; water at 20℃; Stage #2: at 80℃; for 12h; | 90% |

| Conditions | Yield |
|---|---|
| With sodium hydrogen telluride In ethanol 1.) 0 deg C, 2.) 40 deg C, 30 min; | 89% |

| Conditions | Yield |
|---|---|
| In chloroform 1:1 mixt. stirred for 10 min; evapd., washed (Et2O), dried (vac.); NMR; | A 89% B n/a |

-
-
57337-85-6
trimethyl(4-mercaptomethylphenyl)silane

-
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With potassium trimethylsilonate In dimethyl sulfoxide at 60℃; under 760.051 Torr; for 12h; Catalytic behavior; Reagent/catalyst; Solvent; Sealed tube; | 88% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; tiolacetic acid In methanol at 20℃; for 0.5h; | A 87% B 13% |
-
-
26504-29-0
dibenzyl trithiocarbonate

-
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride In toluene at 110℃; | 86% |

| Conditions | Yield |
|---|---|
| With tetraphosphorus decasulfide In toluene for 1.5h; Solvent; Reagent/catalyst; Reflux; | 85% |
| With diethyl ether; ethylmagnesium bromide | |
| With diethyl ether; phenylmagnesium bromide |

| Conditions | Yield |
|---|---|
| Stage #1: benzyl alcohol With sodium sulfide; sodium hydrogencarbonate In ethanol for 2h; Reflux; Enzymatic reaction; Stage #2: With hydrogenchloride In ethanol; water Reagent/catalyst; | 85% |
| Stage #1: benzyl alcohol With N-Bromosuccinimide; triphenylphosphine In acetone at -10 - 25℃; Substitution; Stage #2: With polymer supported hydrosulfide resin In acetone at 25℃; for 0.166667h; Substitution; | 81% |
| With Lawessons reagent In toluene for 48h; Heating; | 55% |
-
-
67-56-1
methanol

-
-
27249-90-7
benzyl dithiobenzoate

-
A
-
5873-86-9
methyl thiobenzoate

-
B
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 1h; | A 82% B n/a |

-
-
42049-35-4
S-benzyl thiocarbamate

-
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| Stage #1: benzyl S-thiocarbamate With tetraphosphorus decasulfide In toluene Reflux; Stage #2: With water Acidic conditions; | 80% |
-
-
6937-97-9
allyl(benzyl)sulfide

-
A
-
26551-50-8
1-benzylsulfanyl-3-chloro-propan-2-ol

-
B
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With water; copper dichloride; lithium tetrachloropalladate(II) In N,N-dimethyl-formamide at 50℃; for 24h; | A 78.7% B 10% |
-
-
61775-34-6
SS-benzyl O-methyl carbono(dithioperoxoate)

-
A
-
72050-07-8
methyl α-(phenylthio)acetate

-
B
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With triphenylphosphine In benzene Ambient temperature; | A 78% B 21% |

| Conditions | Yield |
|---|---|
| With tetraphosphorus decasulfide In toluene for 3h; Solvent; Reflux; | 75% |
-
-
156275-86-4
Benzylsulfanyl-triisopropyl-silane

-
A
-
426-67-5
triisopropylsilyl fluoride

-
B
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride; water In tetrahydrofuran 1.) 25 deg C, 1 h; | A n/a B 73% |
-
-
101561-50-6
benzyl 2-methylprop-2-en-1-yl sulfide

-
A
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With water; copper dichloride; lithium tetrachloropalladate(II) In N,N-dimethyl-formamide at 50℃; for 48h; | A 10% B 72.6% |
-
-
26504-30-3
O,S-dibenzyl dithiocarbonate

-
A
-
538-74-9
dibenzyl sulfide

-
B
-
26504-28-9
dithiocarbonic acid S,S'-dibenzyl ester

-
C
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| Aliquat 336 at 100℃; for 1.5h; Yields of byproduct given; | A n/a B 70% C n/a |


| Conditions | Yield |
|---|---|
| In water; acetic acid electroreduction on Hg-cathode, 0.8 A; | A 70% B 13% |

| Conditions | Yield |
|---|---|
| Stage #1: acetic anhydride With molybdenium(VI) dioxodichloride In dichloromethane at 20℃; for 0.5h; Stage #2: phenylmethanethiol In dichloromethane at 20℃; for 4h; | 100% |
| With silica gel for 0.0533333h; Microwave irradiation; neat (no solvent); | 100% |
| yttria-stabilized zirconia In acetonitrile for 6h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With pyridine chromium peroxide for 0.02h; Product distribution; effect of various chromium(VI) based oxidants; | 100% |
| With barium ferrate(VI) In benzene for 1h; Product distribution; Heating; | 100% |
| With pyridine chromium peroxide for 0.02h; | 100% |
-
-
75-52-5
nitromethane

-
-
108-94-1
cyclohexanone

-
-
100-53-8
phenylmethanethiol

-
-
335458-24-7
1-benzylthio-1-nitromethylcyclohexane

| Conditions | Yield |
|---|---|
| With piperidine In benzene | 100% |
| With piperidine In acetonitrile for 4h; Heating; | 96% |
| With piperidine In acetonitrile for 4h; Heating / reflux; | 22% |
| With piperidine In benzene Heating; |

-
-
75-52-5
nitromethane

-
-
120-92-3
cyclopentanone

-
-
100-53-8
phenylmethanethiol

-
-
335458-23-6
1-benzylthio-1-nitromethylcyclopentane

| Conditions | Yield |
|---|---|
| With piperidine In benzene | 100% |
| With piperidine In acetonitrile for 4h; Heating; | 94% |
| With piperidine In benzene Heating; |

-
-
102-96-5
(2-nitroethenyl)benzene

-
-
100-53-8
phenylmethanethiol

-
-
34980-76-2, 63509-10-4, 63509-11-5
benzyl (2-nitro-1-phenylethyl) sulfide

| Conditions | Yield |
|---|---|
| at 20℃; for 0.1h; Michael addition; neat (no solvent); | 100% |
| at 20℃; for 2h; Michael addition; Neat (no solvent); regioselective reaction; | 92% |
| In water at 20℃; for 1h; thia-Michael addition; | 91% |
| With 2,2'-azobis(isobutyronitrile); quinoclamine; N,N-dimethyl-formamide; Quinine In toluene |
-
-
96-09-3
styrene oxide

-
-
100-53-8
phenylmethanethiol

-
-
117037-28-2
1-(1-phenyl-2-hydroxy) ethyl-benzyl sulfide

| Conditions | Yield |
|---|---|
| With erbium(III) triflate In acetonitrile at 25℃; for 0.75h; | 100% |
| aluminum oxide; molybdenum(VI) oxide at 20℃; for 0.25h; | 89% |
| ammonium cerium(IV) nitrate In acetonitrile for 1.5h; Heating; | 70% |
| With 2C21H12N3O6(3-)*Co(3+)*17H2O*Tb(3+) In neat (no solvent) at 25℃; for 4h; Reagent/catalyst; regioselective reaction; | 86 %Chromat. |
-
-
87-41-2
2-benzofuran-1(3H)-one

-
-
100-53-8
phenylmethanethiol

-
-
1218-59-3
2-<(Benzylthio)methyl>benzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide; mineral oil for 45h; Reflux; | 100% |
| With sodium hydride In N,N-dimethyl-formamide for 24h; Heating; | 99% |
| 58% | |
| With sodium ethanolate In ethanol |

| Conditions | Yield |
|---|---|
| Stage #1: phenylmethanethiol With potassium hydroxide In methanol at 70℃; for 0.25h; Inert atmosphere; Schlenk technique; Green chemistry; Stage #2: benzyl bromide With copper(l) iodide In methanol at 110℃; for 12h; Inert atmosphere; Schlenk technique; Green chemistry; | 100% |
| Stage #1: phenylmethanethiol With sodium methylate In methanol at -10℃; for 0.166667h; Stage #2: benzyl bromide In methanol at 20℃; | 96% |
| Stage #1: phenylmethanethiol With sodium hydride In tetrahydrofuran at 20℃; for 0.5h; Inert atmosphere; Stage #2: benzyl bromide In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; | 92% |
-
-
816-39-7
1,3-dibromoroacetone

-
-
100-53-8
phenylmethanethiol

-
-
19216-97-8
1,3-Bis<(phenylmethyl)thio>-2-propanone

| Conditions | Yield |
|---|---|
| With sodium In ethanol at -5℃; for 4h; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine; O-phenyl phosphorodichloridate In 1,2-dimethoxyethane for 16h; Ambient temperature; | 100% |
-
-
88766-29-4
(methoxycarbonyl)disulfanyl chloride

-
-
100-53-8
phenylmethanethiol

-
-
94839-63-1
Methoxycarbonyl benzyl trisulfane

| Conditions | Yield |
|---|---|
| In dichloromethane at -78℃; for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With pyridine In diethyl ether for 0.5h; | 100% |
-
-
129397-81-5
methyl (((9H-fluoren-9-yl)methoxy)carbonyl)-L-phenylalaninate

-
-
100-53-8
phenylmethanethiol

-
A
-
2577-90-4
methyl (2S)-2-amino-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In N,N-dimethyl-formamide for 0.0333333h; Product distribution; other protected peptides, other thiol, var. TBAF conc., var. time, var. solvent, with or without ultrasound mixing; | A n/a B 100% |
| With tetrabutyl ammonium fluoride In N,N-dimethyl-formamide for 0.05h; | A n/a B 100% |

-
-
34162-79-3
vinylidene-1,1-diphosphonic acid

-
-
100-53-8
phenylmethanethiol

-
-
87774-70-7
(2-Benzylsulfanyl-1-phosphono-ethyl)-phosphonic acid

| Conditions | Yield |
|---|---|
| With acetic acid; triethylamine at 110℃; for 15h; Product distribution; reaction of vinylidenediphosphonic acid with various thiols; | 100% |
| With acetic acid; triethylamine at 110℃; for 15h; | 100% |
-
-
75-52-5
nitromethane

-
-
100-53-8
phenylmethanethiol

-
-
67-64-1
acetone

-
-
105780-12-9
benzyl(2-methyl-1-nitropropan-2-yl)sulfane

| Conditions | Yield |
|---|---|
| With piperidine In benzene | 100% |
| With piperidine In tetrahydrofuran for 12h; Heating; | 96% |
| With piperidine for 15h; Heating; | 49% |
| In benzene at 100℃; for 22h; Dean-Stark; | 23% |
| With piperidine In benzene for 10h; Reflux; Inert atmosphere; |

-
-
100-53-8
phenylmethanethiol

-
-
409108-52-7
Benzylthiosulfenylchlorid

| Conditions | Yield |
|---|---|
| With pyridine; sulfur dichloride In diethyl ether at -78℃; for 0.5h; | 100% |

| Conditions | Yield |
|---|---|
| With benzyl alcohol; aluminium dodecatungsten phosphate In dichloromethane at 20℃; for 4h; | 100% |
| With methanesulfonic acid at 80℃; for 0.0833333h; Microwave irradiation; Ionic liquid; | 99% |
| With boron trifluoride diethyl etherate In dichloromethane at 20℃; for 0.0166667h; | 99% |
| With toluene-4-sulfonic acid In acetonitrile for 12h; Reflux; | 97% |
| With toluene-4-sulfonic acid In benzene |

| Conditions | Yield |
|---|---|
| With sodium carbonate In methanol; water at 20℃; for 0.5h; | 100% |
| With sodium ethanolate In ethanol for 0.166667h; Reflux; Inert atmosphere; | 99% |
| With potassium carbonate In acetonitrile for 3h; Inert atmosphere; | 95% |
-
-
100-53-8
phenylmethanethiol

-
-
176257-53-7
[(3aR,5S,6aR)-5-Azidomethyl-2,2-dimethyl-dihydro-furo[2,3-d][1,3]dioxol-(6Z)-ylidene]-acetic acid methyl ester

| Conditions | Yield |
|---|---|
| With lithium methanolate In methanol for 0.0833333h; | 100% |
-
-
100-53-8
phenylmethanethiol

-
-
251982-26-0
acetic acid 3-acetoxy-6-acetoxymethyl-2-(4,5-diacetoxy-2-acetoxymethyl-6-but-3-enyloxy-tetrahydro-pyran-3-yloxy)-5-(3,4,5-triacetoxy-6-acetoxymethyl-tetrahydro-pyran-2-yloxy)-tetrahydro-pyran-4-yl ester

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile) In 1,4-dioxane at 50 - 80℃; Addition; | 100% |
-
-
100-53-8
phenylmethanethiol

-
-
221636-53-9
N,N-dimethyl-5,7-bis(trifluoroacetyl)-8-quinolylamine

-
-
268745-25-1
1-[8-Benzylsulfanyl-5-(2,2,2-trifluoro-acetyl)-quinolin-7-yl]-2,2,2-trifluoro-ethanone

| Conditions | Yield |
|---|---|
| In acetonitrile for 8h; Substitution; Heating; | 100% |
-
-
4897-68-1
2-iodo-4-methoxybromobenzene

-
-
100-53-8
phenylmethanethiol

-
-
334708-27-9
2-benzylsulfanyl-1-bromo-4-methoxy-benzene

| Conditions | Yield |
|---|---|
| With triethylamine; 1,1'-bis-(diphenylphosphino)ferrocene; bis(dibenzylideneacetone)-palladium(0) In N,N-dimethyl-formamide at 70℃; for 2.5h; | 100% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; triethylamine; bis(dibenzylideneacetone)-palladium(0) In N,N-dimethyl-formamide at 70℃; for 3h; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; N-benzyl-N,N,N-triethylammonium chloride at 20℃; for 1h; | 100% |
-
-
75-52-5
nitromethane

-
-
100-53-8
phenylmethanethiol

-
-
78-93-3
butanone

-
-
335458-22-5
2-benzylthio-2-nitromethylbutane

| Conditions | Yield |
|---|---|
| With piperidine In benzene | 100% |
| With ethylenediamine In acetonitrile for 8h; Heating; | 95% |
| With ethylenediamine In acetonitrile |

-
-
75-52-5
nitromethane

-
-
100-53-8
phenylmethanethiol

-
-
502-42-1
cycloheptanone

-
-
335458-25-8
1-benzylthio-1-nitromethylcycloheptane

| Conditions | Yield |
|---|---|
| With piperidine In benzene | 100% |
| With ethylenediamine In acetonitrile for 12h; Heating; | 83% |

-
-
638-10-8
ethyl 3-methylbut-2-enoate

-
-
100-53-8
phenylmethanethiol

-
-
377092-98-3
ethyl 3-methyl-3-benzylthiobutanoate

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 20 - 50℃; for 36h; | 100% |
-
-
100-53-8
phenylmethanethiol

-
-
161581-95-9
(4-bromo-phenyl)-(2-fluoro-4-methoxy-phenyl)-methanone

-
-
663152-21-4
(2-benzylsulfanyl-4-methoxyphenyl)(4-bromophenyl)methanone

| Conditions | Yield |
|---|---|
| Stage #1: phenylmethanethiol With potassium tert-butylate In tetrahydrofuran at 20℃; for 0.5h; Stage #2: (4-bromo-phenyl)-(2-fluoro-4-methoxy-phenyl)-methanone In tetrahydrofuran at 20℃; for 1.5h; | 100% |
| With potassium tert-butylate In tetrahydrofuran at 20℃; |
-
-
13894-21-8
ethynyl p-tolyl sulfone

-
-
100-53-8
phenylmethanethiol

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 0℃; for 0.333333h; | 100% |
-
-
100-53-8
phenylmethanethiol

-
-
133613-71-5
(4R,5aS,7aS,7bR)-5,5a,6,7,7a,7b-hexahydro-7b-hydroxy-4-methyl-indeno[1,7-bc]furan-2(4H)-one

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 12h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: phenylmethanethiol With tetra-(n-butyl)ammonium iodide; caesium carbonate In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 1-iodo-propane In N,N-dimethyl-formamide at 0 - 20℃; for 2h; | 100% |
Benzyl mercaptan Chemical Properties
Product Name: α-Toluenethiol (CAS NO.100-53-8)
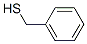
Molecular Formula: C7H8S
Molecular Weight: 124.2g/mol
Mol File: 100-53-8.mol
Einecs: 202-862-5
Appearance: Colourless liquid with a leek or garlic-like odour
Melting Point: -29 °C
Boiling point: 195.3 °C at 760 mmHg
Flash Point: 70 °C
Density: 1.058 g/mL at 25 °C(lit.)
Refractive index: n20/D 1.575(lit.)
Sensitive: Air Sensitive
Surface Tension: 37.2 dyne/cm
Enthalpy of Vaporization: 41.39 kJ/mol
Vapour Pressure: 0.591 mmHg at 25°C
XLogP3: 2.4
H-Bond Donor: 0
H-Bond Acceptor: 0
Product Categories: Phenol and Thiophenol and Mercaptan; thiol Flavor
Benzyl mercaptan Uses
α-Toluenethiol (CAS NO.100-53-8) can be used as pesticides, pharmaceutical intermediates, it can be used as food flavor.
Benzyl mercaptan Production
α-Toluenethiol (CAS NO.100-53-8) is made by benzyl isothiourea hydrochloride plus alkaline hydrolysis, and then dilute sulfuric acid derived. The S-benzyl-isothiourea hydrochloride and 10% sodium hydroxide solution by adding the reactor, using direct fire heating reflux 2h hydrolysis. And then cooled then diluted sulfuric acid acidification, extracted with diethyl ether, ethyl ether extract dried with anhydrous sodium sulfate, filter out the drying agent, recycling ether, the residue vacuum distillation, collecting the boiling point 98-108 ℃ (2.67kPa), the fraction shall be finished.
Benzyl mercaptan Toxicity Data With Reference
| 1. | eye-rbt 106 mg | AIHAAP American Industrial Hygiene Association Journal. 19 (1958),171. | ||
| 2. | orl-rat LD50:493 mg/kg | AIHAAP American Industrial Hygiene Association Journal. 19 (1958),171. | ||
| 3. | ipr-rat LD50:373 mg/kg | AIHAAP American Industrial Hygiene Association Journal. 19 (1958),171. | ||
| 4. | ihl-mus LC50:178 ppm/4H | AIHAAP American Industrial Hygiene Association Journal. 19 (1958),171. | ||
| 5. | ipr-mus LD50:100 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD277-689 . |
Benzyl mercaptan Consensus Reports
Reported in EPA TSCA Inventory.
Benzyl mercaptan Safety Profile
Poison by inhalation and intraperitoneal routes. Moderately toxic by ingestion. An eye irritant. Questionable carcinogen with experimental tumorigenic data. Flammable when exposed to heat or flame. Can react vigorously with oxidizing materials. To fight fire, use foam, CO2, dry chemical, water spray, mist, fog. When heated to decomposition and on contact with acid or acid fumes it emits highly toxic fumes of SOx. See also SULFIDES and MERCAPTANS.
Safety Information of α-Toluenethiol (CAS NO.100-53-8):
Hazard Codes: T![]() ,N
,N.gif)
Risk Statements: 22-23-50/53
22: Harmful if swallowed
23: Toxic by inhalation
50: Very Toxic to aquatic organisms
53: May cause long-term adverse effects in the aquatic environment
Safety Statements: 23-26-36/37/39-45-61-60
23: Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37: Wear suitable gloves
39: Wear eye/face protection
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
60: This material and/or its container must be disposed of as hazardous waste
61: Avoid release to the environment. Refer to special instructions safety data sheet
Benzyl mercaptan Standards and Recommendations
DOT Classification: 3; Label: Flammable Liquid, Poison (UN 1228); DOT Class: 6.1; Label: Poison, Flammable Liquid (UN 3071)
Benzyl mercaptan Specification
α-Toluenethiol ,its CAS NO. is 100-53-8,the synonyms is a-Toluenethiol ; Benzyl mercaptan ; Benzenemethanethiol ; Fema 2147 ; Alpha-toluenethiol ; Alpha-tolyl mercaptan ; Toluenethiol ; Toluene-a-thiol .
Related Products
- Benzyl (1-(aminocarbonyl)-2-hydroxypropyl)carbamate
- Benzyl (1-cyano-1-methylethyl)carbamate
- Benzyl (1R,2S)-3-chloro-2-hydroxy-1-(phenylthiomethyl)propylcarbamate
- Benzyl (3R)-3-aminopiperidine-1-carboxylate
- Benzyl (3R)-piperidin-3-ylcarbamate
- Benzyl (3R,4R,5S)-3-hydroxy-4-(4-hydroxyphenyl)-5-(triisopropylsilanyloxy)piperidine-1-carboxylate
- Benzyl (3S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylate
- Benzyl (R)-5-oxotetrahydrofuran-3-ylcarbamate
- Benzyl (S)-(-)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylate p-toluenesulfonic acid salt
- Benzyl [(3S)-2,5-dioxopyrrolidin-3-yl]carbamate
- 100538-35-0
- 1005-38-5
- 1005-39-6
- 10054-21-4
- 100545-61-7
- 1005474-88-3
- 100-54-9
- 100-55-0
- 100553-83-1
- 100556-58-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View