-
Name
Benzyladenine
- EINECS 214-927-5
- CAS No. 1214-39-7
- Article Data54
- CAS DataBase
- Density 1.393 g/cm3
- Solubility soluble in water
- Melting Point 229-233 °C
- Formula C12H11N5
- Boiling Point 529.388 °C at 760 mmHg
- Molecular Weight 225.253
- Flash Point 273.964 °C
- Transport Information UN 2348 3/PG 3
- Appearance white to light yellow crystalline powder
- Safety 9-24/25-36/37/39-26-23
- Risk Codes 36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms 6-Benzylamino Purine;6-Benzylaminopurine ( 6-BA);Benzylaminopurine;6-Benzylaminopurine (6BA);6-Benzyl Adenine(6-Benzyl Aminopurine);6-Benzylaminopurine;BA;6-Benzylaminopurine *;6-Benzylamino purine (6-BA);N-benzyl-7H-purin-6-amine;Paturyl;N-Benzyl-9H-purin-6-amine;N-(Phenylmethyl)-1H-purin-6-amine;BA (growth stimulator);6-(Benzylamino)purine;Prestwick_414;6-Benzylaminopurine (6-BA);N6-Benzyladenine;BAP (growth stimulant);N(6)-Benzylaminopurine;1H-Purin-6-amine,N-(phenylmethyl)-;Aminopurine, 6-benzyl;6-Benzyl Aminopurine;6-benzylaminopurine BA;6-(n-Benzyl) Aminopurin;N-6-Benzyladenine;benzyl(purin-6-yl)amine;9H-Purin-6-amine, N-(phenylmethyl)-;
- PSA 66.49000
- LogP 2.03800
Synthetic route

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 5h; Reflux; | 96% |
| at 100℃; for 0.0833333h; microwave irradiation; | 95% |
| With indium(III) chloride In acetonitrile at 120℃; for 1h; Microwave irradiation; regioselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 100℃; for 3h; Reagent/catalyst; Temperature; | 92% |

-
-
111098-24-9
Benzotriazol-1-ylmethyl-(9H-purin-6-yl)-amine

-
-
1214-39-7
6-benzyladenine

| Conditions | Yield |
|---|---|
| In diethyl ether at 25℃; for 16h; | 80% |

| Conditions | Yield |
|---|---|
| In butan-1-ol for 0.75h; Substitution; | 78.2% |

-
-
1214-39-7
6-benzyladenine

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrogen; palladium on activated charcoal In ethanol under 760 Torr; for 15h; Hydrogenation; | 69% |

| Conditions | Yield |
|---|---|
| at 130℃; for 17h; | 55% |
-
-
7387-58-8, 80007-24-5
6-N-2',3',5'-tri-O-tetraacetyladenosine

-
A
-
1214-39-7
6-benzyladenine

-
B
-
18646-11-2
α-D-ribofuranosyl-1-phosphate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / N,N-dimethyl-formamide / 20 h / 20 °C 2: methanol; ammonia / 48 h / 20 °C 3: potassium dihydrogenphosphate; Escherichia coli purine nucleoside phosphorylase / 37 °C / pH 7.5 / aq. buffer; Enzymatic reaction View Scheme |
-
-
1338578-76-9
N6-acetyl-2',3',5'-tri-O-acetyl-N6-benzyladenosine

-
A
-
1214-39-7
6-benzyladenine

-
B
-
18646-11-2
α-D-ribofuranosyl-1-phosphate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: methanol; ammonia / 48 h / 20 °C 2: potassium dihydrogenphosphate; Escherichia coli purine nucleoside phosphorylase / 37 °C / pH 7.5 / aq. buffer; Enzymatic reaction View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: potassium carbonate / N,N-dimethyl-formamide / 20 h / 20 °C 2: methanol; ammonia / 48 h / 20 °C 3: potassium dihydrogenphosphate; Escherichia coli purine nucleoside phosphorylase / 37 °C / pH 7.5 / aq. buffer; Enzymatic reaction View Scheme |

| Conditions | Yield |
|---|---|
| With Escherichia coli purine nucleoside phosphorylase at 25℃; pH=7.5; Kinetics; aq. phosphate buffer; Enzymatic reaction; | |
| With potassium dihydrogenphosphate; Escherichia coli purine nucleoside phosphorylase at 37℃; pH=7.5; Equilibrium constant; aq. buffer; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: magnesium methanolate / methanol / 3 h / 55 °C 2: sodium tetrahydroborate / methanol / 20 °C View Scheme |
-
-
709619-15-8
C12H9N5

-
-
1214-39-7
6-benzyladenine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 20℃; |

-
-
1214-39-7
6-benzyladenine

| Conditions | Yield |
|---|---|
| With propylamine In methanol at 20℃; |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 75℃; for 4h; Temperature; Reagent/catalyst; | 18.2 g |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: bis(trichloromethyl) carbonate; dmap; thionyl chloride / Reflux; Green chemistry 2: triethylamine / ethanol / Reflux; Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: trichlorophosphate; N,N-dimethyl-aniline / 760.05 Torr / Heating 2: water / 0.75 h / 100 °C / Green chemistry View Scheme |
-
-
59278-00-1
2-acetoxyethyl acetoxymethyl ether

-
-
1214-39-7
6-benzyladenine

-
-
173205-63-5
9-<(2-acetoxyethoxy)methyl>-6-benzylaminopurine

| Conditions | Yield |
|---|---|
| With aluminum oxide; silica gel for 0.0666667h; microwave irradiation; | 95% |

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole In dimethyl sulfoxide at 70℃; for 2h; Michael addition; | 94% |
-
-
107-04-0
1-Bromo-2-chloroethane

-
-
1214-39-7
6-benzyladenine

-
-
120593-22-8
6-benzylamino-9-(2-chloroethyl)purine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; (C8H17)4NBr In water; benzene at 80℃; for 0.5h; Product distribution; other catalyst, other solvent, other reaction time, other temperature; | 90% |
| With sodium hydroxide; (C8H17)4NBr In water; benzene at 80℃; for 0.5h; | 90% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; | 82% |
-
-
292638-85-8
acrylic acid methyl ester

-
-
1214-39-7
6-benzyladenine

| Conditions | Yield |
|---|---|
| With 1-butyl-3-methylimidazolium hydroxide at 25℃; for 2h; Michael addition; | 90% |
| With 1-methyl-1H-imidazole In dimethyl sulfoxide at 70℃; for 2h; Michael addition; | 84% |
-
-
141-32-2
acrylic acid n-butyl ester

-
-
1214-39-7
6-benzyladenine

-
-
1033400-03-1
3-(6-benzylamino-purin-9-yl)-propionic acid butyl ester

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; zinc(II) oxide at 120℃; for 0.333333h; Michael addition; microwave irradiation; | 89% |
-
-
109-70-6
1,3-chlorobromopropane

-
-
1214-39-7
6-benzyladenine

-
-
120593-24-0
6-benzylamino-9-(3-chloropropyl)purine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; (C8H17)4NBr In water; benzene at 80℃; for 1h; | 85% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 0.0833333h; microwave irradiation; | 85% |
-
-
106-93-4
ethylene dibromide

-
-
1214-39-7
6-benzyladenine

-
-
120593-27-3
6-benzylamino-9-(2-bromoethyl)purine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; | 82% |
| With sodium hydroxide; tetrabutylammomium bromide In dichloromethane; water at 40℃; for 1h; Product distribution; other reaction time, other temperature; | 50% |
| With sodium hydroxide; tetrabutylammomium bromide In dichloromethane; water at 40℃; for 1h; | 50% |
-
-
107-04-0
1-Bromo-2-chloroethane

-
-
1214-39-7
6-benzyladenine

-
-
120593-26-2
Benzyl-(9-vinyl-9H-purin-6-yl)-amine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; (C8H17)4NBr In water; benzene at 80℃; for 2.5h; Product distribution; other catalyst, other solvent, other reaction time, other temperature; | 80% |
| With sodium hydroxide; (C8H17)4NBr In water; benzene at 80℃; for 2.5h; | 80% |
-
-
1214-39-7
6-benzyladenine

-
-
2032-35-1
Bromoacetaldehyde diethyl acetal

-
-
1527480-25-6
N-benzyl-9-(2,2-diethoxyethyl)-9H-purin-6-amine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 8h; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 160℃; for 0.133333h; microwave irradiation; | 78% |

| Conditions | Yield |
|---|---|
| With D-aminoacylase from Escherichia coli (EC 3.5.1.81) In dimethyl sulfoxide at 50℃; for 96h; | 77% |
| With [bmIm]OH at 50℃; for 24h; Markovnikov addition; | 70% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 160℃; for 0.133333h; microwave irradiation; | 76% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In dimethyl sulfoxide at 140℃; | 76% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; (C8H17)4NBr In benzene for 3h; Heating; | 75% |
| With potassium carbonate In dimethyl sulfoxide Alkylation; |
-
-
68673-84-7
methyl 1,2,3-tri-O-acetyl-β-D-ribofuronate

-
-
1214-39-7
6-benzyladenine

-
-
1012864-67-3
methyl 1-[N6-(benzyl)adenin-9-yl]-2,3-di-O-acetyl-β-D-ribofuronate

| Conditions | Yield |
|---|---|
| Stage #1: 6-benzyladenine With ammonium sulfate; 1,1,1,3,3,3-hexamethyl-disilazane for 16h; Heating; Stage #2: methyl 1,2,3-tri-O-acetyl-β-D-ribofuronate With trimethylsilyl trifluoromethanesulfonate In 1,2-dichloro-ethane at 90℃; for 0.333333h; microwave irradiation; Further stages.; | 75% |

-
-
166826-77-3
(carboxymethyl(4-methylbenzyl)amino)acetic acid

-
-
7732-18-5
water

-
-
1214-39-7
6-benzyladenine

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 25 - 50℃; for 1h; | 75% |


| Conditions | Yield |
|---|---|
| With triethylamine In water at 100℃; for 0.0833333h; Michael addition; microwave irradiation; | 74% |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
1214-39-7
6-benzyladenine

-
-
2312-73-4
6-benzylamino-9-(2-tetrahydropyranyl)purine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In various solvent(s) for 2h; Addition; Heating; | 71.4% |
-
-
110-80-5
2-ethoxy-ethanol

-
-
1214-39-7
6-benzyladenine

-
-
1013022-32-6
benzyl-[9-(2-ethoxy-ethyl)-9H-purin-6-yl]-amine

| Conditions | Yield |
|---|---|
| With iodine; potassium carbonate; triethylamine; triphenylphosphine In N,N-dimethyl-formamide for 9h; Reflux; | 71% |
| With potassium carbonate; 1-n-butyl-3-methylimidazolim bromide; triethylamine; p-toluenesulfonyl chloride at 80℃; for 7h; Green chemistry; regioselective reaction; | 64% |
| With potassium carbonate; triethylamine In N,N-dimethyl-formamide for 4h; Heating; | 58% |
| With tetrachloromethane; tetra-(n-butyl)ammonium iodide; potassium carbonate; triphenylphosphine In N,N-dimethyl-formamide for 5h; Reflux; | 58% |

-
-
57362-11-5
2,2'-((furan-2-ylmethyl)azanediyl)diacetic acid

-
-
1214-39-7
6-benzyladenine

| Conditions | Yield |
|---|---|
| In methanol; water at 25℃; for 0.5h; | 70% |


-
-
1214-39-7
6-benzyladenine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 24h; regioselective reaction; | A 3% B 70% |

Benzyladenine Consensus Reports
Reported in EPA TSCA Inventory.
Benzyladenine Specification
The Benzyladenine, with the CAS registry number 1214-39-7, is also known as Benzyl(purin-6-yl)amine. It belongs to the product categories of Pharmaceutical Raw Materials; Miscellaneous; Purine; Amines; Biochemistry; Cytokinins; Nucleobases and their analogs; Nucleosides, Nucleotides & Related Reagents; Plant Growth Regulators; Plant Hormones. Its EINECS number is 214-927-5. This chemical's molecular formula is C12H11N5 and molecular weight is 225.25. What's more, its systematic name is N-Benzyl-7H-purin-6-amine. Its classification codes are: (1)Agricultural Chemical; (2)Growth Substances; (3)Growth regulator / Fertilizer; (4)Mutation data; (5)Plant growth regulators. This chemical should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides. This substance is a first-generation synthetic cytokinin that elicits plant growth and development responses, setting blossoms and stimulating fruit richness by stimulating cell division. It is an inhibitor of respiratory kinase in plants, and increases post-harvest life of green vegetables.
Physical properties of Benzyladenine are: (1)ACD/LogP: 2.164; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.15; (4)ACD/LogD (pH 7.4): 2.16; (5)ACD/BCF (pH 5.5): 25.36; (6)ACD/BCF (pH 7.4): 25.92; (7)ACD/KOC (pH 5.5): 349.77; (8)ACD/KOC (pH 7.4): 357.43; (9)#H bond acceptors: 5; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 66.49 Å2; (13)Index of Refraction: 1.766; (14)Molar Refractivity: 66.899 cm3; (15)Molar Volume: 161.701 cm3; (16)Polarizability: 26.521×10-24cm3; (17)Surface Tension: 80.4 dyne/cm; (18)Density: 1.393 g/cm3; (19)Flash Point: 273.964 °C; (20)Enthalpy of Vaporization: 80.442 kJ/mol; (21)Boiling Point: 529.388 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation: this chemical can be prepared by 7(9)H-purin-6-ylamine and phenylmethanol; sodium salt at the temperature of 130 °C. The reaction time is 17 hours. The yield is about 55%.
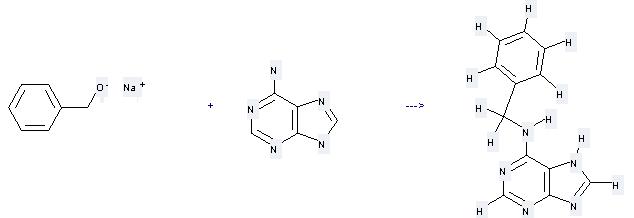
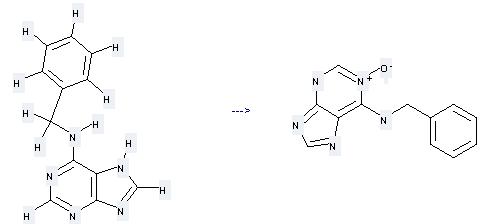
Uses of Benzyladenine: it can be used to produce by benzyl-(1-oxy-9H-purin-6-yl)-amine at the temperature of 30 °C. It will need regent MCPBA and solvent methanol with the reaction time of 20 hours. The yield is about 35%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation, in contact with skin and if swallowed. It is irritating to eyes, respiratory system and skin. You must avoid contact with skin and eyes. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. You should keep the container in a well-ventilated place. You must not breathe gas/fumes/vapour/spray (appropriate wording to be specified by the manufacturer). When using it, you need wear suitable protective clothing, gloves and eye/face protection.
You can still convert the following datas into molecular structure:
(1)SMILES: n1c(c2c(nc1)ncn2)NCc3ccccc3
(2)Std. InChI: InChI=1S/C12H11N5/c1-2-4-9(5-3-1)6-13-11-10-12(15-7-14-10)17-8-16-11/h1-5,7-8H,6H2,(H2,13,14,15,16,17)
(3)Std. InChIKey: NWBJYWHLCVSVIJ-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 1300mg/kg (1300mg/kg) | SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 19, Pg. 336, 1972. |
| mouse | LD50 | skin | > 5gm/kg (5000mg/kg) | Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 19, Pg. 336, 1972. | |
| mouse | LD50 | subcutaneous | > 2300mg/kg (2300mg/kg) | Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 19, Pg. 336, 1972. | |
| rat | LD50 | oral | 2125mg/kg (2125mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) SENSE ORGANS AND SPECIAL SENSES: LACRIMATION: EYE | Toho Igakkai Zasshi. Journal of Medical Society of Toho University. Vol. 19, Pg. 336, 1972. |
Related Products
- Benzyladenine
- 121442-74-8
- 121442-75-9
- 121443-79-6
- 121445-21-4
- 121-44-8
- 12145-00-5
- 12145-48-1
- 121-45-9
- 121-46-0
- 12146-93-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View