-
Name
Chlorocyclohexane
- EINECS 208-806-6
- CAS No. 542-18-7
- Article Data304
- CAS DataBase
- Density 1 g/mL at 25 °C(lit.)
- Solubility 0.02 g/L (20 ºC)
- Melting Point - 44 °C
- Formula C6H11Cl
- Boiling Point 142 °C at 760 mmHg
- Molecular Weight 118.606
- Flash Point 28.9 °C
- Transport Information UN 1993 3/PG 3
- Appearance clear liquid
- Safety 26-36-37/39-16
- Risk Codes 10-36/37/38-20
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi, Xn
Xn
- Synonyms chlor-cyclohexane;4-05-00-00048 (Beilstein Handbook Reference);1/C6H11Cl/c7-6-4-2-1-3-5-6/h6H,1-5H;cyclohexane, chloro-;Monochlorocyclohexane;Cyclohexyl Chloride;
- PSA 0.00000
- LogP 2.55790
Synthetic route
-
-
32308-83-1
nonafluoro-tert-butanesulfenyl chloride

-
-
110-82-7
cyclohexane

-
A
-
32308-82-0
nonafluoro-tert-butanethiol

-
B
-
542-18-7
cyclohexyl chloride

| Conditions | Yield |
|---|---|
| A 100% B 100% | |
| A 100% B 100% |


| Conditions | Yield |
|---|---|
| With bismuth(III) chloride In tetrachloromethane for 0.0833333h; Heating; | 99% |
| With 1,2,3-Benzotriazole; thionyl chloride In dichloromethane at 20℃; Substitution; | 92% |
| With triphenylphosphine; zinc(II) chloride; diethylazodicarboxylate In tetrahydrofuran for 2h; Ambient temperature; | 90% |

| Conditions | Yield |
|---|---|
| With bismuth(III) chloride In 1,2-dichloro-ethane for 0.25h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tetrabutyl-ammonium chloride; sodium chloride In water at 20℃; for 5h; Reagent/catalyst; | 97% |
| With hydrogenchloride; potassium chloride; tetrabutyl-ammonium chloride In water at 20℃; Irradiation; Green chemistry; | 95% |
| With sodium hypochlorite In water; acetone at 20℃; for 2h; Reagent/catalyst; Solvent; Time; Inert atmosphere; | 93% |
-
-
110-82-7
cyclohexane

-
-
2712-93-8
dichlorofluoromethanesulphenyl chloride

-
A
-
542-18-7
cyclohexyl chloride

-
B
-
675-63-8
bis(dichlorofluoromethyl)disulfane

-
C
-
68409-02-9
Dichlorfluormethyl-cyclohexylsulfid

| Conditions | Yield |
|---|---|
| A 63.6% B 97% C 3% | |
| A 63.6% B 97% C 3% |


| Conditions | Yield |
|---|---|
| With ethanol; trichlorovinylsilane; tin(IV) chloride for 1h; Ambient temperature; | 90% |
| With dichloromethane; RhCl(PPh3)3 | 72% |
| With chloro-trimethyl-silane; water for 15h; Ambient temperature; | 70% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 105℃; for 3h; | 90% |
| With boron trichloride In hexane at 0℃; for 0.0833333h; | 78% |
| With diethylaluminium chloride In hexane at -78℃; for 0.166667h; | 74 % Chromat. |

| Conditions | Yield |
|---|---|
| With CPT | A n/a B 90% |
-
-
993-38-4
chlorodifluoromethansulfenylchloride

-
-
110-82-7
cyclohexane

-
A
-
542-18-7
cyclohexyl chloride

-
B
-
692-58-0
bis(chlorodifluoromethyl)disulfane

-
C
-
68409-01-8
(Chloro-difluoro-methylsulfanyl)-cyclohexane

-
D
-
68409-06-3
benzyl chloro-difluoromethyl sulfide

| Conditions | Yield |
|---|---|
| A 39.4% B 88.4% C 11.6% D 65% | |
| A 39.4% B 88.4% C 11.6% D 65% |


-
-
542-18-7
cyclohexyl chloride

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; tetrachloromethane In water; tert-butyl alcohol for 4h; Irradiation; | 86% |
-
-
662-42-0
heptafluoro-propane-1-sulphenic acid chloride

-
-
110-82-7
cyclohexane

-
A
-
356-07-0
bis(perfluoro-n-propyl)disulfane

-
B
-
542-18-7
cyclohexyl chloride

-
C
-
68409-00-7
heptafluoro-n-propyl cyclohexyl sulfide

| Conditions | Yield |
|---|---|
| A 85.7% B 43.2% C 14.3% | |
| A 85.7% B 43.2% C 14.3% | |
| for 0.1h; Mechanism; Irradiation; effect of bis(trifluoromethyl) disulfide; |

-
-
931-56-6
2-methoxycyclohexane

-
A
-
542-18-7
cyclohexyl chloride

-
B
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With phenylacetyl chloride; zinc In Petroleum ether at 28℃; for 4h; Yields of byproduct given; | A 84% B n/a |
-
-
931-56-6
2-methoxycyclohexane

-
-
103-80-0
phenylacetyl chloride

-
A
-
542-18-7
cyclohexyl chloride

-
B
-
101-41-7
benzeneacetic acid methyl ester

| Conditions | Yield |
|---|---|
| With zinc In Petroleum ether at 28℃; for 4h; Yields of byproduct given; | A n/a B 84% |


| Conditions | Yield |
|---|---|
| With 1-chloro-1-(dimethylamino)-2-methyl-1-propene In dichloromethane 1.) 0 deg C; 2.) rt., 3h; | A 18 % Spectr. B 82% |
| With hydrogenchloride | |
| With aluminium trichloride Erwaermen auf dem Dampfbad; |

-
A
-
2527-66-4
2-methyl-1,2-benzisothiazole-3(2H)-one

-
B
-
542-18-7
cyclohexyl chloride

-
D
-
110-83-8
cyclohexene

| Conditions | Yield |
|---|---|
| With thionyl chloride In dichloromethane for 1h; Mechanism; Product distribution; Heating; also pyrolysis in toluene, 110 deg C, 20 h, without SOCl2; further o-(N-methylcarbamoyl)phenyl sulphoxides; | A 81% B 11% C 16% D 67% |
| With thionyl chloride In dichloromethane for 1h; Heating; | A 81% B 11% C 16% D 67% |

-
-
33427-17-7
(1R,2R)-1-Chloro-2-iodo-cyclohexane

-
-
542-18-7
cyclohexyl chloride

| Conditions | Yield |
|---|---|
| With 1,2-bis(diphenylphosphino)ethane nickel(II) chloride; tri-n-butyl-tin hydride In tetrahydrofuran for 0.25h; Ambient temperature; | 80% |
-
-
110-82-7
cyclohexane

-
-
114541-87-6
benzyloxychlorocarbene

-
A
-
542-18-7
cyclohexyl chloride

-
B
-
100-44-7
benzyl chloride

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| at 25℃; Photolysis; | A 9% B 80% C 11% |


| Conditions | Yield |
|---|---|
| In benzene at 30℃; Kinetics; Solvent; Temperature; Inert atmosphere; Schlenk technique; | A n/a B 70% |
-
-
110-82-7
cyclohexane

-
-
98-85-1, 13323-81-4
1-Phenylethanol

-
A
-
542-18-7
cyclohexyl chloride

-
B
-
10017-37-5
tert-butylamine hydrochloride

-
C
-
98-86-2
acetophenone

| Conditions | Yield |
|---|---|
| With N,N-dichloro-t-butylamine at 20℃; for 1h; Irradiation; | A 68% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With dichloromethylsilane; iron(III) chloride In 1,2-dimethoxyethane for 6h; Heating; | 66% |
| Multi-step reaction with 2 steps 1: diethyl ether; sodium; alcohol / Reagens 4: Kaliumcarbonat 2: fuming hydrochloric acid / 100 °C View Scheme | |
| Stage #1: cyclohexanone With iron(III) chloride In ethyl acetate at 20℃; for 0.0166667h; Stage #2: With dimethylmonochlorosilane In ethyl acetate at 20℃; |
-
-
110-82-7
cyclohexane

-
-
51031-50-6
heptafluoropropane-2-sulphenic acid chloride

-
A
-
542-18-7
cyclohexyl chloride

-
B
-
754-62-1
bis(perfluoroisopropyl) disulfide

-
C
-
68408-97-9
heptafluoropropane-2-thiol

-
D
-
68408-99-1
Heptafluorisopropylcyclohexylsulfid

| Conditions | Yield |
|---|---|
| A 37.7% B 65% C n/a D 0.5% | |
| A 37.7% B 65% C n/a D 0.5% |

-
-
98-07-7
Benzotrichlorid

-
-
108-93-0
cyclohexanol

-
A
-
98-87-3
benzylidene dichloride

-
B
-
542-18-7
cyclohexyl chloride

| Conditions | Yield |
|---|---|
| With TOP; phenylsilane In neat (no solvent) at 100℃; for 24h; Appel Halogenation; Inert atmosphere; | A n/a B 57% |


| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; (FeCl2(C6H6N2CO)(CH3)2(C5H4N)2(C(O)OCH3)2); tetrabutyl-ammonium chloride In acetonitrile at 25℃; for 0.583333h; Inert atmosphere; chemoselective reaction; | A 5.1% B 55.4% |
| With [FeIV(1,1-di(pyridin-2-yl)-N,N-bis(quinolin-2-ylmethyl)methanamine)(O)]2+; [FeII(1,1-di(pyridin-2-yl)-N,N-bis(quinolin-2-ylmethyl)methanamine)(Cl)]Cl In acetonitrile at 25℃; for 0.5h; Inert atmosphere; Glovebox; | A 52% B n/a |
| With 5,10,15,20-tetraphenyl-21 H,23-H-porphine manganese(III)chloride; iodosylbenzene In dichloromethane; water for 12h; | A 18% B 30% |
-
-
421-17-0
Trifluoromethylsulfenyl chloride

-
-
110-82-7
cyclohexane

-
A
-
372-64-5
Bis(trifluoromethyl)disulfid

-
B
-
6476-52-4
cyclohexyl(trifluoromethyl)sulfane

-
C
-
542-18-7
cyclohexyl chloride

| Conditions | Yield |
|---|---|
| byproducts: HCl; Irradiation (UV/VIS); | A 35% B 45% C 28% |
| byproducts: HCl; Irradiation (UV/VIS); | A 35% B 45% C 28% |
| for 0.2h; Mechanism; Irradiation; effect of bis(heptafluoro-n-propyl) disulfide; |

-
-
56-34-8
tetraethylammonium chloride

-
-
937-14-4
3-chloro-benzenecarboperoxoic acid

-
A
-
542-18-7
cyclohexyl chloride

-
B
-
1128-76-3
3-chloro-benzoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With cyclohexane In acetonitrile at 20℃; for 3h; Yields of byproduct given; | A n/a B 38% |

-
-
110-82-7
cyclohexane

-
A
-
542-18-7
cyclohexyl chloride

-
B
-
108-94-1
cyclohexanone

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With chloroform; pyrimido<5,4-g>pteridine N-oxide for 3h; Product distribution; Mechanism; Irradiation; | A 11% B 12% C 37% |
| With [oxoiron(IV)(tris-(quinolyl-2-methyl)amine)(chloride)](1+) In [D3]acetonitrile at -40℃; Reagent/catalyst; | A 35% B 3% C 7% |
| With barium hexaferrite; acetic acid; magnesium chloride In dichloromethane at 23℃; for 2h; Product distribution; Further Variations:; Reagents; | A 31% B 13% C 10% |


| Conditions | Yield |
|---|---|
| With potassium permanganate; aluminium trichloride In acetonitrile for 0.5h; | A 35% B 15% |
| With [oxoiron(IV)(tris(pyridyl-2-methyl)amine)(chloride)](triflate) In [D3]acetonitrile at 25℃; Inert atmosphere; | A 11% B 2% |
| With cis-VI(6,6'-Cl2bpy)2O2> In tetrachloromethane; acetonitrile at 20℃; for 0.5h; Oxidation; | A 0.5 % Chromat. B 50 % Chromat. |
| With peracetic acid; triethylamine hydrochloride; acetic acid In acetonitrile at 22℃; Inert atmosphere; sealed system; |
-
-
56-23-5
tetrachloromethane

-
-
110-83-8
cyclohexene

-
A
-
542-18-7
cyclohexyl chloride

-
-
7484-12-0, 55756-67-7, 62435-49-8
trans-1-trichloromethyl-2-chlorocyclohexane

| Conditions | Yield |
|---|---|
| With tris(triphenylphosphine)ruthenium(II) chloride; water at 150℃; for 6h; Autoclave; | A n/a B 25% C 25% |

-
-
110-82-7
cyclohexane

-
A
-
542-18-7
cyclohexyl chloride

-
B
-
15619-54-2
tert-butyl cyclohexyl peroxide

-
C
-
108-94-1
cyclohexanone

-
D
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With Co(III)(BPI)(OAc)(OO-tBu) at 60℃; for 2h; Product distribution; stoichiometric oxidation by various cobalt(III)alkylperoxy complexes, solvents; | A n/a B 18% C 24% D 18% |

-
-
75-91-2
tert.-butylhydroperoxide

-
-
110-82-7
cyclohexane

-
-
107-13-1
acrylonitrile

-
A
-
542-18-7
cyclohexyl chloride

-
B
-
75354-09-5
2-Chlor-3-cyclohexylpropiononitril

-
C
-
158750-03-9
2-tert-Butylperoxy-3-cyclohexyl-propionitrile

| Conditions | Yield |
|---|---|
| With acetic acid; ferric nitrate; copper dichloride In pyridine at 60℃; Product distribution; Mechanism; further with LiCl; | A 15.8% B 8.7% C 3.1% |


| Conditions | Yield |
|---|---|
| With hydrogen bromide; ferric(III) bromide In dichloromethane at 25℃; for 0.3h; | 99% |
| With hydrogen bromide at 105℃; for 4h; | 90% |
| With ferric(III) bromide; hydrogen bromide In dichloromethane at 25℃; for 2h; | |
| With hydrogen bromide; cetyltributylphosphonium bromide at 105℃; for 7h; | 80 % Chromat. |
| With ferric(III) bromide; hydrogen bromide In dichloromethane at 25℃; for 0.3h; Product distribution; Mechanism; other alkyl chlorides; other hydrogen halide; also without HBr; var. reaction conditions; | 99 % Chromat. |
-
-
542-18-7
cyclohexyl chloride

-
-
15769-84-3
N-<(S)-1-phenylethyl>-2-pyridinecarboxamide

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; ethylmagnesium bromide; manganese(ll) chloride In tetrahydrofuran at 60℃; for 14h; Catalytic behavior; Reagent/catalyst; Schlenk technique; Inert atmosphere; chemoselective reaction; | 99% |
-
-
542-18-7
cyclohexyl chloride

-
-
13139-86-1
4-methoxyphenyl magnesium bromide

-
-
613-36-5
4-cyclohexylanisole

| Conditions | Yield |
|---|---|
| With iron(III) chloride; N,N′-bis(2,6-diisopropylphenyl)imidazol-2-ylidene hydrochloride In tetrahydrofuran at 0 - 40℃; for 1.66667h; | 98% |
| Stage #1: cyclohexyl chloride With [1,3-bis(3,5-di-tert-butyl-2-hydroxybenzyl)benzimidazolium][FeCl4] In diethyl ether at 0℃; for 0.0333333h; Schlenk technique; Inert atmosphere; Stage #2: 4-methoxyphenyl magnesium bromide In diethyl ether at 0 - 30℃; for 2h; Temperature; Schlenk technique; Inert atmosphere; | 86% |
| With iron(III) chloride; triethylamine In diethyl ether for 0.5h; Grignard cross-coupling reaction; Heating; |

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide In acetonitrile at 20℃; for 4h; Reagent/catalyst; Inert atmosphere; UV-irradiation; | 98% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride at 0 - 20℃; for 4h; Friedel-Crafts Alkylation; | 95% |
| With aluminum (III) chloride at 3 - 20℃; |
-
-
542-18-7
cyclohexyl chloride

-
-
352-13-6
4-flourophenylmagnesium bromide

-
-
1717-84-6
1-cyclohexyl-4-fluorobenzene

| Conditions | Yield |
|---|---|
| With iron(III) chloride; N,N′-bis(2,6-diisopropylphenyl)imidazol-2-ylidene hydrochloride In tetrahydrofuran at 0 - 40℃; for 1.66667h; | 95% |
| Stage #1: cyclohexyl chloride With [1,3-bis(3,5-di-tert-butyl-2-hydroxybenzyl)benzimidazolium][FeCl4] In diethyl ether at 0℃; for 0.0333333h; Schlenk technique; Inert atmosphere; Stage #2: 4-flourophenylmagnesium bromide In diethyl ether at 0 - 30℃; for 2h; Temperature; Schlenk technique; Inert atmosphere; | 78% |
-
-
542-18-7
cyclohexyl chloride

-
-
1521266-22-7
1-(4-butoxyphenyl)-1-cyclohexyl-3-[4-(2,5-dimethylphenyl)piperazin-1-yl]-2-phenylpropan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: cyclohexyl chloride With magnesium In diethyl ether Stage #2: With 1-(4-butoxyphenyl)-3-[4-(2,5-dimethylphenyl)piperazin-1-yl]-2-phenylpropan-1-one In diethyl ether for 6h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With zinc perchlorate In neat (no solvent) at 80℃; for 3h; Ritter Amidation; | 94% |
| With iron(II) chloride tetrahydrate In neat (no solvent) at 80℃; for 4h; Ritter Amidation; | 94% |
-
-
199477-91-3
N-(2-phenylethyl)pyridine-2-carboxamide

-
-
542-18-7
cyclohexyl chloride

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; ethylmagnesium bromide; manganese(ll) chloride In tetrahydrofuran at 60℃; for 14h; Schlenk technique; Inert atmosphere; chemoselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; C21H23ClNiP2S; caesium carbonate; sodium iodide In dimethyl sulfoxide at 50℃; for 12h; Sonogashira Cross-Coupling; Schlenk technique; Inert atmosphere; | 94% |
-
-
14159-55-8
2-cyclohex-1-enyl-pyridine

-
-
542-18-7
cyclohexyl chloride

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; neopentylmagnesium bromide; cobalt(II) bromide; 1,3-diisopropyl-1H-benzo[d]imidazol-3-ium bromide In tetrahydrofuran at 0 - 20℃; for 6h; Catalytic behavior; Reagent/catalyst; Temperature; Schlenk technique; | 92% |
-
-
542-18-7
cyclohexyl chloride

-
-
10354-51-5
N-butyl-pyridine-2-carboxylic acid amide

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; ethylmagnesium bromide; manganese(ll) chloride In tetrahydrofuran at 60℃; for 14h; Schlenk technique; Inert atmosphere; chemoselective reaction; | 92% |
-
-
542-18-7
cyclohexyl chloride

-
-
886-06-6
1-phenyl-3-piperidino-propan-1-one; hydrochloride

-
-
52-49-3
trihexylphenidyl hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: cyclohexyl chloride With iodine; magnesium In 1,2-dimethoxyethane; tert-butyl methyl ether at 45 - 55℃; for 2h; Sealed tube; Inert atmosphere; Large scale; Stage #2: 1-phenyl-3-piperidino-propan-1-one; hydrochloride In 1,2-dimethoxyethane; tert-butyl methyl ether at 5 - 10℃; for 2h; Solvent; Sealed tube; Inert atmosphere; Large scale; | 90.5% |
| Stage #1: cyclohexyl chloride With magnesium In tetrahydrofuran; ethyl bromide at 45 - 65℃; for 3h; Stage #2: 1-phenyl-3-piperidino-propan-1-one; hydrochloride In tetrahydrofuran; ethyl bromide at 65℃; for 4h; Stage #3: With hydrogenchloride In tetrahydrofuran; ethyl bromide; water at 65℃; for 0.8h; Temperature; | 70.3% |

| Conditions | Yield |
|---|---|
| With hydrogen iodide; cetyltributylphosphonium bromide at 105℃; for 7h; | 90% |
| With hydrogen iodide at 105℃; for 2.5h; | 85% |
| With hydrogen iodide |
-
-
542-18-7
cyclohexyl chloride

-
-
111757-80-3
(-)-(2S)-trifluoromethanesulfonyloxy-propionic acid tert-butyl ester

-
-
1059044-01-7
(+)-(2S)-cyclohexyl-propionic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| Stage #1: cyclohexyl chloride With magnesium In tetrahydrofuran Inert atmosphere; Stage #2: (-)-(2S)-trifluoromethanesulfonyloxy-propionic acid tert-butyl ester With zinc(II) chloride In tetrahydrofuran at 0℃; for 3h; Inert atmosphere; optical yield given as %ee; enantiospecific reaction; | 90% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride at 0 - 20℃; for 4h; Friedel-Crafts Alkylation; | 90% |
| With aluminum (III) chloride at 0 - 20℃; for 5h; | 90% |
-
-
542-18-7
cyclohexyl chloride

-
-
4294-57-9
para-methylphenylmagnesium bromide

-
-
4501-36-4
1-cyclohexyl-4-methylbenzene

| Conditions | Yield |
|---|---|
| Stage #1: cyclohexyl chloride With [1,3-bis(3,5-di-tert-butyl-2-hydroxybenzyl)benzimidazolium][FeCl4] In diethyl ether at 0℃; for 0.0333333h; Schlenk technique; Inert atmosphere; Stage #2: para-methylphenylmagnesium bromide In diethyl ether at 0 - 30℃; for 2h; Temperature; Reagent/catalyst; Schlenk technique; Inert atmosphere; | 89% |
| With C33H49N2O2(1+)*Cl4Fe(1-) In diethyl ether Reagent/catalyst; Grignard Reaction; | 77% |
| With butylmethylimidazolium tetrachloroferrate In diethyl ether at 0 - 20℃; | 75% |

| Conditions | Yield |
|---|---|
| With potassium fluoride on basic alumina In 2-methyltetrahydrofuran at 90℃; for 12h; Inert atmosphere; chemoselective reaction; | 89% |
-
-
124-38-9
carbon dioxide

-
-
542-18-7
cyclohexyl chloride

-
-
62-53-3
aniline

-
-
3770-95-4
O-cyclohexyl N-phenylcarbamate

| Conditions | Yield |
|---|---|
| With choline chloride * 2ZnCl2 at 20℃; under 760.051 Torr; for 2h; Green chemistry; | 89% |


| Conditions | Yield |
|---|---|
| With oxygen; kieselguhr; copper(l) chloride In hexane for 3h; Oxidation; Heating; | 88% |
| Stage #1: cyclohexyl chloride With sodium hydroxide In water at 45 - 50℃; for 2.5h; Stage #2: With quinolinium chlorochromate(VI) In water for 18h; | 37% |
| With pumice stone; vanadia at 350℃; beim Leiten im Gemisch mit Luft und Wasserdampf; |
-
-
542-18-7
cyclohexyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: cyclohexyl chloride With naphthalene; lithium In tetrahydrofuran at -78℃; for 3h; Stage #2: (2SP,4R,5S)-(-)-3,4-dimethyl-2,5-diphenyl-1,3,2-oxazaphospholidine borane In tetrahydrofuran at -78℃; Further stages.; | 88% |
| Stage #1: cyclohexyl chloride With lithium In Petroleum ether for 5h; Heating; Stage #2: (2SP,4R,5S)-(-)-3,4-dimethyl-2,5-diphenyl-1,3,2-oxazaphospholidine borane In tetrahydrofuran; Petroleum ether at -78 - 0℃; | 87% |
Chlorocyclohexane Chemical Properties
Molecule structure of Chlorocyclohexane (CAS NO.542-18-7):
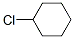
IUPAC Name: Chlorocyclohexane
Molecular Formula: C6H11Cl
Molecular Weight: 118.62 g/mol
Density: 1 g/mL at 25 °C(lit.)
Melting Point: -44 °C
Boiling Point: 142 °C at 760 mmHg
Flash Point: 28.9 °C
Storage Temp.: Flammables area
Water Solubility: 0.02 g/L (20 °C)
Index of Refraction: 1.451
Molar Refractivity: 32.58 cm3
Molar Volume: 120.9 cm3
Surface Tension: 27.7 dyne/cm
XLogP3: 3
Exact Mass: 118.054928
MonoIsotopic Mass: 118.054928
Canonical SMILES: C1CCC(CC1)Cl
InChI: InChI=1S/C6H11Cl/c7-6-4-2-1-3-5-6/h6H,1-5H2
InChIKey: UNFUYWDGSFDHCW-UHFFFAOYSA-N
EINECS: 208-806-6
Product Categories: CHLORO ALKANE COMPOUNDS
Chlorocyclohexane Uses
Chlorocyclohexane (CAS NO.542-18-7) is used as pharmaceutical intermediates. It is also used in organic synthesis.
Chlorocyclohexane Toxicity Data With Reference
| 1. | ihl-rat LCLo:31 g/m3 | GTPZAB Gigiena Truda i Professionalnye Zabolevaniia. Labor Hygiene and Occupational Diseases. 10 (1)(1966),49. | ||
| 2. | ihl-mus LC50:31 g/m3/2H | 85GMAT Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure Izmerov, N.F., et al.,Moscow, USSR.: Centre of International Projects, GKNT,1982,36. |
Chlorocyclohexane Consensus Reports
Reported in EPA TSCA Inventory.
Chlorocyclohexane Safety Profile
Hazard Codes:  Xi,
Xi,  Xn
Xn
Risk Statements: 10-36/37/38-20
R10:Flammable.
R36/37/38:Irritating to eyes, respiratory system and skin.
R20:Harmful by inhalation.
Safety Statements: 26-36-37/39-16
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
S37/39:Wear suitable gloves and eye/face protection.
S16:Keep away from sources of ignition.
RIDADR: UN 1993 3/PG 3
WGK Germany: 1
RTECS: GU8685000
HazardClass: 3
PackingGroup: III
HS Code: 29035990
Slightly toxic by inhalation. When heated to decomposition it emits toxic vapors of Cl−.
Chlorocyclohexane Specification
Chlorocyclohexane (CAS NO.542-18-7) is also named as 4-05-00-00048 (Beilstein Handbook Reference) ; AI3-23841 ; BRN 1900796 ; Cyclohexyl chloride ; HSDB 2801 ; Monochlorocyclohexane ; NSC 8434 ; Cyclohexane, chloro- . Chlorocyclohexane (CAS NO.542-18-7) is clear liquid.
Related Products
- Chlorocyclohexane
- 5421-92-1
- 5422-00-4
- 5422-17-3
- 54221-93-1
- 54221-95-3
- 54221-96-4
- 54223-20-0
- 5422-34-4
- 5422-35-5
- 5422-44-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View