-
Name
Cylopropylmethyl chloride
- EINECS 227-632-1
- CAS No. 5911-08-0
- Article Data35
- CAS DataBase
- Density 1.056 g/cm3
- Solubility Not miscible in water.
- Melting Point -91 °C
- Formula C4H7Cl
- Boiling Point 87.6 °C at 760 mmHg
- Molecular Weight 90.5526
- Flash Point 29 °F
- Transport Information UN 1993 3/PG 2
- Appearance COA
- Safety 16-26-36/37/39
- Risk Codes 11-36/37/38
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xi
Xi
- Synonyms (Chloromethyl)cyclopropane;Chlorocyclopropylmethane;Cyclopropanemethyl chloride;Cyclopropylcarbinylchloride;Cyclopropylchloromethane;a-(Chloromethyl)cyclopropane;
- PSA 0.00000
- LogP 1.63520
Synthetic route

| Conditions | Yield |
|---|---|
| With phenylphosphorus tetrachloride In chloroform at 0℃; for 0.0833333h; | 98% |
| With 1,1,1,3,3,3-hexachloro-propan-2-one; triphenylphosphine at 0 - 20℃; for 3.25h; | 95% |
| With 1,3,5-trichloro-2,4,6-triazine In acetonitrile at -10 - 25℃; for 5h; Solvent; Temperature; | 94% |
-
-
56-23-5
tetrachloromethane

-
-
112057-32-6
tri(cyclopropylmethyl)phosphite

-
A
-
927-73-1
3-butenyl chloride

-
B
-
27301-78-6
Phosphoric acid tricyclopropylmethyl ester

-
C
-
112142-26-4
Trichloromethyl-phosphonic acid dicyclopropylmethyl ester

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| In toluene at 80℃; Further byproducts given; | A 16% B 2% C 80% D 64% |

-
-
2516-33-8
Cyclopropylmethanol

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| A 15% B 18% C 80% |

-
-
2154-76-9
cyclopropylmethyl

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1190-22-3
1,3-dichlorobutane

-
C
-
1790-22-3
1,2,4-trichlorobutane

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| With chlorine In benzene Rate constant; Product distribution; Irradiation; other chlorinating agents; | A 10.3% B 6.2% C 19.1% D 34.4% |

-
-
594-11-6
methylcyclopropane

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1190-22-3
1,3-dichlorobutane

-
C
-
1790-22-3
1,2,4-trichlorobutane

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| With chlorine In 1,1,2-Trichloro-1,2,2-trifluoroethane at 20℃; Irradiation; | A 10.3% B 6.2% C 19.1% D 34.4% |
| With tert-butylhypochlorite In 1,1,2-Trichloro-1,2,2-trifluoroethane at 20℃; for 2.5h; Irradiation; | A 17% B 3.2% C 6.5% D 24.2% |


| Conditions | Yield |
|---|---|
| With phosphorus pentachloride | |
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With chlorine Irradiation; | |
| With chlorine at -196.1℃; Quantum yield; Irradiation; var. magnetic field; |
-
-
1120-57-6
chlorocyclobutane

-
-
127-08-2
potassium acetate

-
-
64-19-7
acetic acid

-
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| at 100℃; Rate constant; Solvolyse und Isomerisierung; |

-
-
186581-53-3, 908094-01-9
diazomethane

-
-
107-05-1
3-chloroprop-1-ene

-
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| Cu catalyst |
-
-
310447-99-5, 310448-01-2
3,4-dichlorobut-1-ene

-
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| (i) 9-bora-bicyclo<3.3.1>nonane, THF, (ii) aq. NaOH; Multistep reaction; |
-
-
56-23-5
tetrachloromethane

-
-
112057-32-6
tri(cyclopropylmethyl)phosphite

-
A
-
927-73-1
3-butenyl chloride

-
B
-
6554-31-0
Dicyclopropylcarbinyl-phosphonat

-
C
-
27301-78-6
Phosphoric acid tricyclopropylmethyl ester

-
D
-
112142-26-4
Trichloromethyl-phosphonic acid dicyclopropylmethyl ester

-
E
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| In toluene at 80℃; for 4h; Product distribution; Mechanism; | A 16 % Chromat. B 2 % Chromat. C 2 % Chromat. D 80 % Chromat. E 64 % Chromat. |

-
-
64-17-5
ethanol

-
-
122651-94-9
3-cyclopropylmethoxy-3-chlorodiaziridine

-
A
-
927-73-1
3-butenyl chloride

-
B
-
44611-46-3
allylcarbinyl ethyl ether

-
C
-
1120-57-6
chlorocyclobutane

-
D
-
70097-82-4
cyclobutyl ethyl ether

-
E
-
70097-81-3
cyclopropylcarbinyl ethyl ether

-
F
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| In ethanol; acetonitrile at 25℃; Thermodynamic data; Kinetics; Product distribution; Ea, effect of solvents; |

-
-
64-17-5
ethanol

-
-
122651-94-9
3-cyclopropylmethoxy-3-chlorodiaziridine

-
A
-
44611-46-3
allylcarbinyl ethyl ether

-
B
-
70097-82-4
cyclobutyl ethyl ether

-
C
-
70097-81-3
cyclopropylcarbinyl ethyl ether

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| at 25℃; Further byproducts given; | A 1 % Chromat. B 22 % Chromat. C 28 % Chromat. D 42 % Chromat. |

-
-
64-17-5
ethanol

-
-
122651-94-9
3-cyclopropylmethoxy-3-chlorodiaziridine

-
A
-
1120-57-6
chlorocyclobutane

-
B
-
70097-82-4
cyclobutyl ethyl ether

-
C
-
70097-81-3
cyclopropylcarbinyl ethyl ether

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| at 25℃; Further byproducts given; | A 5 % Chromat. B 22 % Chromat. C 28 % Chromat. D 42 % Chromat. |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate; mercury dichloride 1.) CH2Cl2, 20 deg C, 2.) 20 deg C; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; |
-
-
75637-38-6
cyclopropylcarbinol hypochlorite

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| With triphenyl phosphite In dichloromethane at -78℃; | A 1.5 % Chromat. B 8.5 % Chromat. C 90 % Chromat. |

-
-
75637-39-7
cyclobutyl hypochlorite

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| With triphenyl phosphite In dichloromethane at -78℃; | A 5 % Chromat. B 29 % Chromat. C 66 % Chromat. |

-
-
122651-94-9
3-cyclopropylmethoxy-3-chlorodiaziridine

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; | A 7 % Chromat. B 15 % Chromat. C 78 % Chromat. |
| With tetrabutylammonium tetrafluoroborate In acetonitrile at 25℃; | A 9 % Chromat. B 27 % Chromat. C 64 % Chromat. |
| With tetrabutylammonium tetrafluoroborate In acetonitrile at 25℃; | A 9 % Chromat. B 27 % Chromat. C 64 % Chromat. |

-
-
594-11-6
methylcyclopropane

-
-
7782-50-5
chlorine

-
A
-
927-73-1
3-butenyl chloride

-
B
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| at -20℃; | |
| at 50℃; |

-
-
7719-09-7
thionyl chloride

-
-
2516-33-8
Cyclopropylmethanol

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

-
-
110-86-1
pyridine

-
-
7719-09-7
thionyl chloride

-
-
2516-33-8
Cyclopropylmethanol

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

-
-
7719-09-7
thionyl chloride

-
-
2919-23-5
cyclobutanol

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

-
-
110-86-1
pyridine

-
-
7719-09-7
thionyl chloride

-
-
2919-23-5
cyclobutanol

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

-
-
64-17-5
ethanol

-
A
-
1120-57-6
chlorocyclobutane

-
B
-
70097-82-4
cyclobutyl ethyl ether

-
C
-
70097-81-3
cyclopropylcarbinyl ethyl ether

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| at 25℃; Product distribution; Further Variations:; Solvents; |

-
-
64-17-5
ethanol

-
-
364612-99-7
cyclobutoxychlorocarbene

-
A
-
1120-57-6
chlorocyclobutane

-
B
-
70097-82-4
cyclobutyl ethyl ether

-
C
-
70097-81-3
cyclopropylcarbinyl ethyl ether

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| at 25℃; Product distribution; Further Variations:; Solvents; |


| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; Product distribution; Kinetics; Further Variations:; Solvents; | |
| In 1,2-dichloro-ethane at 25℃; Kinetics; | |
| In acetonitrile at 25℃; Product distribution; Further Variations:; Solvents; | A 10 % Chromat. B 19 % Chromat. C 71 % Chromat. |

-
-
364612-99-7
cyclobutoxychlorocarbene

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane at 25℃; Kinetics; | |
| In acetonitrile at 25℃; Product distribution; Kinetics; Further Variations:; Solvents; | |
| In acetonitrile at 25℃; Product distribution; Further Variations:; Solvents; | A 10 % Chromat. B 35 % Chromat. C 55 % Chromat. |

-
-
122651-94-9
3-cyclopropylmethoxy-3-chlorodiaziridine

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
110-56-5
1,4-dichlorobutane

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| at 365℃; Product distribution; | A 4.9 % Chromat. B 22.9 % Chromat. C 28.9 % Chromat. D 43.4 % Chromat. |

-
-
225668-16-6
5,6-bis(3-fluoro-4-methoxyphenyl)-2H-pyridazin-3-one

-
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| 100% |
-
-
91060-92-3
1-(4-hydroxy-2,6-dimethylphenyl)ethanone

-
-
5911-08-0
chloro(cyclopropyl)methane

-
-
1439935-91-7
1-(4-(cyclopropylmethoxy)-2,6-dimethylphenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 16h; Inert atmosphere; | 99% |
-
-
767-00-0
4-cyanophenol

| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; potassium carbonate In N,N-dimethyl-formamide at 50℃; for 24h; | 98% |
-
-
22246-16-8
6-nitro-3,4-dihydro-1H-quinolin-2-one

-
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| Stage #1: 6-nitro-3,4-dihydro-1H-quinolin-2-one With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Inert atmosphere; Stage #2: chloro(cyclopropyl)methane With potassium iodide In N,N-dimethyl-formamide at 120℃; for 2h; Inert atmosphere; | 94% |
| Stage #1: 6-nitro-3,4-dihydro-1H-quinolin-2-one With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Inert atmosphere; Stage #2: chloro(cyclopropyl)methane With potassium iodide In N,N-dimethyl-formamide at 120℃; for 2h; | 94% |
| With potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 120℃; for 2h; Inert atmosphere; | 94% |
| Stage #1: 6-nitro-3,4-dihydro-1H-quinolin-2-one With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 0.0833333h; Inert atmosphere; Stage #2: chloro(cyclopropyl)methane With potassium iodide In N,N-dimethyl-formamide at 120℃; Inert atmosphere; | 94% |
-
-
225668-09-7
5-(3-fluoro-4-methoxyphenyl)-6-(4-methoxyphenyl)-2H-pyridazin-3-one

-
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| 93.8% |
-
-
14648-14-7
N-norcodeine hydrochloride

-
-
5911-08-0
chloro(cyclopropyl)methane

-
-
77794-51-5
17-(cyclopropylmethyl)-7,8-didehydro-4,5α-epoxy-3-methoxymorphinan-6α-ol

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In N,N-dimethyl-formamide at 100℃; for 18h; | 93% |
-
-
225668-02-0
6-(3-fluoro-4-methoxyphenyl)-5-(4-methoxyphenyl)-2H-pyridazine-3-one

-
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| 93% |
-
-
142529-02-0
11-oxo(10H)-pyrrolo<2,1-c><1,2,5>benzothiadiazepine 5,5-dioxide

-
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetone for 24h; Ambient temperature; | 90% |

| Conditions | Yield |
|---|---|
| With 1-Phenylprop-1-yne; copper dichloride In tetrahydrofuran at 70℃; for 12h; | 90% |
-
-
5911-08-0
chloro(cyclopropyl)methane

-
-
1070294-27-7
6-bromo-2-(2-cyclopropanemethyl)isoquinolin-1(2H)-one

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 50℃; for 3h; | 90% |
-
-
5911-08-0
chloro(cyclopropyl)methane

-
-
70768-88-6
5,6-bis(4-Methoxyphenyl)-4-ethoxy-carbonyl-2H-pyridazin-3-one

-
-
288586-95-8
5,6-bis(4-methoxyphenyl)-2-cyclopropylmethyl-4-ethoxycarbonyl-2H-pyridazin-3-one

| Conditions | Yield |
|---|---|
| 89.1% |

| Conditions | Yield |
|---|---|
| Stage #1: (1r,4r)-4-(tritylamino)cyclohexanol With sodium hydride In tetrahydrofuran at 0℃; for 1h; Stage #2: chloro(cyclopropyl)methane In tetrahydrofuran at 20℃; for 6h; | 86.4% |
-
-
5911-08-0
chloro(cyclopropyl)methane

-
-
159022-20-5
(-)-17-(cyclopropylmethyl)-3,14β-dimethoxy-4,5-α-epoxy-5β-methylmorphinan-6-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 22h; | 86% |
-
-
5911-08-0
chloro(cyclopropyl)methane

-
-
209808-01-5
AR-M390

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 25℃; for 0.5h; | 86% |

| Conditions | Yield |
|---|---|
| With phenylphosphorus tetrachloride In chloroform at 0℃; for 0.0833333h; | 98% |
| With 1,1,1,3,3,3-hexachloro-propan-2-one; triphenylphosphine at 0 - 20℃; for 3.25h; | 95% |
| With 1,3,5-trichloro-2,4,6-triazine In acetonitrile at -10 - 25℃; for 5h; Solvent; Temperature; | 94% |
-
-
56-23-5
tetrachloromethane

-
-
112057-32-6
tri(cyclopropylmethyl)phosphite

-
A
-
927-73-1
3-butenyl chloride

-
B
-
27301-78-6
Phosphoric acid tricyclopropylmethyl ester

-
C
-
112142-26-4
Trichloromethyl-phosphonic acid dicyclopropylmethyl ester

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| In toluene at 80℃; Further byproducts given; | A 16% B 2% C 80% D 64% |

-
-
2516-33-8
Cyclopropylmethanol

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| A 15% B 18% C 80% |

-
-
2154-76-9
cyclopropylmethyl

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1190-22-3
1,3-dichlorobutane

-
C
-
1790-22-3
1,2,4-trichlorobutane

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| With chlorine In benzene Rate constant; Product distribution; Irradiation; other chlorinating agents; | A 10.3% B 6.2% C 19.1% D 34.4% |

-
-
594-11-6
methylcyclopropane

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1190-22-3
1,3-dichlorobutane

-
C
-
1790-22-3
1,2,4-trichlorobutane

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| With chlorine In 1,1,2-Trichloro-1,2,2-trifluoroethane at 20℃; Irradiation; | A 10.3% B 6.2% C 19.1% D 34.4% |
| With tert-butylhypochlorite In 1,1,2-Trichloro-1,2,2-trifluoroethane at 20℃; for 2.5h; Irradiation; | A 17% B 3.2% C 6.5% D 24.2% |


| Conditions | Yield |
|---|---|
| With phosphorus pentachloride | |
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With chlorine Irradiation; | |
| With chlorine at -196.1℃; Quantum yield; Irradiation; var. magnetic field; |
-
-
1120-57-6
chlorocyclobutane

-
-
127-08-2
potassium acetate

-
-
64-19-7
acetic acid

-
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| at 100℃; Rate constant; Solvolyse und Isomerisierung; |

-
-
186581-53-3, 908094-01-9
diazomethane

-
-
107-05-1
3-chloroprop-1-ene

-
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| Cu catalyst |
-
-
310447-99-5, 310448-01-2
3,4-dichlorobut-1-ene

-
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| (i) 9-bora-bicyclo<3.3.1>nonane, THF, (ii) aq. NaOH; Multistep reaction; |
-
-
56-23-5
tetrachloromethane

-
-
112057-32-6
tri(cyclopropylmethyl)phosphite

-
A
-
927-73-1
3-butenyl chloride

-
B
-
6554-31-0
Dicyclopropylcarbinyl-phosphonat

-
C
-
27301-78-6
Phosphoric acid tricyclopropylmethyl ester

-
D
-
112142-26-4
Trichloromethyl-phosphonic acid dicyclopropylmethyl ester

-
E
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| In toluene at 80℃; for 4h; Product distribution; Mechanism; | A 16 % Chromat. B 2 % Chromat. C 2 % Chromat. D 80 % Chromat. E 64 % Chromat. |

-
-
64-17-5
ethanol

-
-
122651-94-9
3-cyclopropylmethoxy-3-chlorodiaziridine

-
A
-
927-73-1
3-butenyl chloride

-
B
-
44611-46-3
allylcarbinyl ethyl ether

-
C
-
1120-57-6
chlorocyclobutane

-
D
-
70097-82-4
cyclobutyl ethyl ether

-
E
-
70097-81-3
cyclopropylcarbinyl ethyl ether

-
F
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| In ethanol; acetonitrile at 25℃; Thermodynamic data; Kinetics; Product distribution; Ea, effect of solvents; |

-
-
64-17-5
ethanol

-
-
122651-94-9
3-cyclopropylmethoxy-3-chlorodiaziridine

-
A
-
44611-46-3
allylcarbinyl ethyl ether

-
B
-
70097-82-4
cyclobutyl ethyl ether

-
C
-
70097-81-3
cyclopropylcarbinyl ethyl ether

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| at 25℃; Further byproducts given; | A 1 % Chromat. B 22 % Chromat. C 28 % Chromat. D 42 % Chromat. |

-
-
64-17-5
ethanol

-
-
122651-94-9
3-cyclopropylmethoxy-3-chlorodiaziridine

-
A
-
1120-57-6
chlorocyclobutane

-
B
-
70097-82-4
cyclobutyl ethyl ether

-
C
-
70097-81-3
cyclopropylcarbinyl ethyl ether

-
D
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| at 25℃; Further byproducts given; | A 5 % Chromat. B 22 % Chromat. C 28 % Chromat. D 42 % Chromat. |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate; mercury dichloride 1.) CH2Cl2, 20 deg C, 2.) 20 deg C; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; |
-
-
75637-38-6
cyclopropylcarbinol hypochlorite

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| With triphenyl phosphite In dichloromethane at -78℃; | A 1.5 % Chromat. B 8.5 % Chromat. C 90 % Chromat. |

-
-
75637-39-7
cyclobutyl hypochlorite

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| With triphenyl phosphite In dichloromethane at -78℃; | A 5 % Chromat. B 29 % Chromat. C 66 % Chromat. |

-
-
122651-94-9
3-cyclopropylmethoxy-3-chlorodiaziridine

-
A
-
927-73-1
3-butenyl chloride

-
B
-
1120-57-6
chlorocyclobutane

-
C
-
5911-08-0
chloro(cyclopropyl)methane

| Conditions | Yield |
|---|---|
| In acetonitrile at 25℃; | A 7 % Chromat. B 15 % Chromat. C 78 % Chromat. |
| With tetrabutylammonium tetrafluoroborate In acetonitrile at 25℃; | A 9 % Chromat. B 27 % Chromat. C 64 % Chromat. |
| With tetrabutylammonium tetrafluoroborate In acetonitrile at 25℃; | A 9 % Chromat. B 27 % Chromat. C 64 % Chromat. |

Cylopropylmethyl chloride Chemical Properties
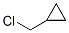
IUPAC Name:chloromethylcyclopropane
Molecular Formula:C4H7Cl
Molecular Weight:90.551380 g/mol
Appearance:colorless liquid
Melting Point:-91°C
Boiling Point:87-89 °C(lit.)
Flash Point:29 °F
Water Solubility:1165 mg/L at 25 °C
Density:0.98 g/mL at 25 °C(lit.)
Refractive index:n20/D 1.435(lit.)
BRN:635697
EINECS:227-632-1
Synonyms of Cylopropylmethyl chloride(5911-08-0):
CYCLOPROPYLMETHYL CHLORIDE;(CHLOROMETHYL)CYCLOPROPANE;Cyclopropane, (chloromethyl)-;Cyclopropane,(chloromethyl);Cylopropylmethylchloride;Cyclopropylmethychloride;Cyclopropylmethyl Chloride, Pract.;CPMCl
Categories of Cylopropylmethyl chloride(5911-08-0):
Cycloalkanes;Alkyl;Halogenated Hydrocarbons;Organic Building Blocks
Cylopropylmethyl chloride Safety Profile
Hazard Codes:F
 ,Xi
,Xi 
Risk Statements:11-36/37/38
11:Highly Flammable
36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements:16-26-36/37/39
16:Keep away from sources of ignition - No smoking
26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36/37/39:Wear suitable protective clothing, gloves and eye/face protection
RIDADR:UN 1993 3/PG 2
WGK Germany:3
HazardClass:3
PackingGroup:II
HS Code:29035990
Cylopropylmethyl chloride Specification
First Aid Measures of Cylopropylmethyl chloride(5911-08-0):
Ingestion:If victim is conscious and alert, give 2-4 cupfuls of milk or water. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:Get medical aid immediately. Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Skin:Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Remove contaminated clothing and shoes.
Eyes:Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Storage of Cylopropylmethyl chloride(5911-08-0):
Keep away from heat, sparks, and flame. Flammables-area.
Related Products
- Cylopropylmethyl chloride
- 59112-78-6
- 591-12-8
- 59113-36-9
- 591-17-3
- 591-18-4
- 59118-78-4
- 59119-18-5
- 591-19-5
- 591-20-8
- 59121-84-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View