-
Name
DIMETHYLZINC
- EINECS 208-884-1
- CAS No. 544-97-8
- Article Data60
- CAS DataBase
- Density 0.92 g/mL at 20 °C
- Solubility soluble in alkanes
- Melting Point -42 °C
- Formula C2H6Zn
- Boiling Point 44-46 °C
- Molecular Weight 95.4596
- Flash Point 30 °F
- Transport Information UN 3399 4.3/PG 2
- Appearance colorless mobile liquid, with peculiar garlic odor
- Safety 26-45-61-62-60-43-36/37/39-33-29-16
- Risk Codes 11-34-50/53-65-67-63-48/20-17-14/15
-
Molecular Structure
-
Hazard Symbols
 F,
F, C,
C, N
N
- Synonyms Dimethylzink;Methylzinc;
- PSA 0.00000
- LogP 1.16510
Synthetic route

| Conditions | Yield |
|---|---|
| at 75℃; for 6.5h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| at 90℃; for 7h; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| at 60℃; for 6h; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With copper(l) iodide In neat (no solvent) CuI, Zn were placed in reactor, evacuated for 10 min, filled with Ar, Al-compound and ligand were added, heated to 42°C for 12 h, cooled to room temp.; vac. distilled; | 90% |

| Conditions | Yield |
|---|---|
| In dibutyl ether soln. MeMgI in n-Bu2O was slowly added to ZnCl2 under vigorous stirring,suspn. was heated at 60°C for 60 h; ZnMe2 was collected by distillation in temp. range 130-160°C; | 75% |
-
-
1000391-90-1
[Zn(Pd(N(FC6H3P(C3H7)2)2))2]

-
-
74-88-4
methyl iodide

-
-
798541-83-0
Pd(CH3)(N(C6H3(F)P(CH(CH3)2)2)2)

-
B
-
544-97-8
dimethyl zinc(II)

| Conditions | Yield |
|---|---|
| In benzene-d6 Irradiation (UV/VIS); at room temp. up to 5 d; NMR; | A n/a B 60% |


| Conditions | Yield |
|---|---|
| In tetrahydrofuran under Ar; THF soln. of MeLi and germylzinc stirred at room temp. for 1.5h; | A 0% B 50% C 50% |
| In diethyl ether germylzinc compd. reacted with 1 mol Li compd.; symmetrical compds. yielded in quantitative reaction in ratio of 1:1 (detmd. by (1)H NMR); | A 0% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With diethyl ether; zinc(II) chloride |
-
-
557-20-0
diethylzinc

-
-
143-36-2
methylmercury(II) iodide

-
A
-
544-97-8
dimethyl zinc(II)

-
B
-
627-44-1
diethylmercury


| Conditions | Yield |
|---|---|
| With zinc at 120℃; |

| Conditions | Yield |
|---|---|
| Durch thermische Zersetzung unter vermindertem Druck und Einw. des entstandenen Methyls auf metallisches Zink; |
-
-
18815-73-1
methylzinc iodide

-
-
544-97-8
dimethyl zinc(II)

| Conditions | Yield |
|---|---|
| at 25℃; |

| Conditions | Yield |
|---|---|
| With sodium amalgam; acetic acid ester; zinc man verwendet einen kupfernen Kessel und destilliert aus dem Oelbad bei moeglichst niedriger Temperatur; | |
| With zinc at 150 - 160℃; | |
| With diethyl ether; zinc at 100℃; Destillation des Reaktionsproduktes; |

-
-
544-97-8
dimethyl zinc(II)

| Conditions | Yield |
|---|---|
| With zinc at 100℃; Destillation des Reaktionsproduktes; |

| Conditions | Yield |
|---|---|
| at 120℃; |

-
-
557-20-0
diethylzinc

-
-
143-36-2
methylmercury(II) iodide

-
A
-
544-97-8
dimethyl zinc(II)

-
B
-
627-44-1
diethylmercury


| Conditions | Yield |
|---|---|
| With zinc Destillation des entstehenden intermediaeren Methylzinkjodid; |

| Conditions | Yield |
|---|---|
| In dodecane excess of Me3Al in dodecane adding to suspn. of anhyd. Zn acetate in dodecane at room temp.; mixt. distg. through a 40 cm vigreux column and product collecting in toluene; identified by b. p.; |

| Conditions | Yield |
|---|---|
| With copper; methyl iodide In neat (no solvent) sealed tube (vac.), Zn/Cu powder contg. 91 % Zn, 373 K (20 h); cooling to 77 K, opening, fractional distn. (vac.); | |
| With hydrogen; copper(II) oxide; methyl iodide In dichloromethane heating Zn and CuO under H2 (color change from black to gray), purging with N2, cooling to room temp., slow addn. of CH3I via the dropping funnel, heating at 65-70°C for abbout 18 h, allowing to come to room temp., cooling to -78°C; whole operation in a hood; Liebig cooler with flasks (CH2Cl2), flasks are cooled (dry ice/acetone bath), distn. at atmospheric pressure, heating of flask (oil bath, 200°C); | 70-75 |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether (Ar); stirring (0°C, 30 min.); | |
| In tetrahydrofuran-d8 under Ar; to mixt. of ZnCl2 and MeLi addded THF-D8 in NMR tube at low temp., allowed to warm to room temp., stirred for 5 min, not isolated; | |
| In diethyl ether (inert atmosphere); not isolated; | |
| In tetrahydrofuran; diethyl ether at 0℃; for 0.5h; Schlenk technique; |

-
-
544-97-8
dimethyl zinc(II)

| Conditions | Yield |
|---|---|
| With methyl iodide In neat (no solvent) reaction of MeI with CuZn (excess of Zn); |
-
-
506-82-1
dimethylcadmium

-
-
32955-11-6
ethylzinc(II) iodide

-
A
-
544-97-8
dimethyl zinc(II)

-
B
-
55204-73-4
methylethylcadmium

| Conditions | Yield |
|---|---|
| In gaseous matrix components vaporization into He, mixt. diln. further with He or H2, equilibration within 20 s in IR cell; IR spectroscopic monitoring; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) (exclusion of moisture); co-condensation of MeZnBH4 and NH3, maintainingthe mixture at -78°C for ca. 24 h, removal of excess NH3 at -78. degree.C, slow warming to room temp. under continuous pumping; elem. anal.; |

-
-
7440-66-6
zinc

-
-
74-95-3
1,2-dibromomethane

-
A
-
92601-82-6
bis(bromomethyl)zinc

-
B
-
544-97-8
dimethyl zinc(II)

-
C
-
4109-95-9
bromomethylzinc bromide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; tetrahydrofuran-d8 byproducts: ethylene, methyl bromide; 35-40°C, molar ratio of Zn:CH2Br2 is 1:1 to 1:4; |

-
-
55204-73-4
methylethylcadmium

-
-
32955-11-6
ethylzinc(II) iodide

-
A
-
544-97-8
dimethyl zinc(II)

-
B
-
592-02-9
diethylcadmium

| Conditions | Yield |
|---|---|
| In gaseous matrix components vaporization into He, mixt. diln. further with He or H2, equilibration within 20 s in IR cell; IR spectroscopic monitoring; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) (exclusion of moisture); 190 K, continuous pumping; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether at 20℃; for 0.75h; | |
| In diethyl ether for 2h; Reflux; Inert atmosphere; | |
| In diethyl ether |

| Conditions | Yield |
|---|---|
| In (2)H8-toluene at 26.84℃; Equilibrium constant; Solvent; Inert atmosphere; Schlenk technique; Glovebox; |


| Conditions | Yield |
|---|---|
| (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In 1,4-dioxane for 2h; Heating; | 100% |
| Stage #1: 1-Bromonaphthalene; dimethyl zinc(II) With n-butyllithium In tetrahydrofuran; diethyl ether; hexane at 0℃; for 0.5h; Substitution; Stage #2: With oxovanadium(V) ethoxydichloride In tetrahydrofuran; diethyl ether; hexane at 20℃; for 3h; Oxidation; Alkylation; Further stages.; | 73% |
-
-
124-38-9
carbon dioxide

-
-
544-97-8
dimethyl zinc(II)

-
-
536-74-3
phenylacetylene

-
-
704-80-3
(E)-3-phenyl-2-butenoic acid

| Conditions | Yield |
|---|---|
| Stage #1: carbon dioxide; phenylacetylene With bis(1,5-cyclooctadiene)nickel (0); 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 0℃; for 1h; Stage #2: dimethyl zinc(II) In tetrahydrofuran; hexane at 0℃; for 2h; Further stages.; | 100% |


| Conditions | Yield |
|---|---|
| (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In 1,4-dioxane for 2h; Heating; | 100% |
-
-
2142-69-0
2-bromophenyl methyl ketone

-
-
544-97-8
dimethyl zinc(II)

-
-
577-16-2, 122382-54-1
2-Methylacetophenone

| Conditions | Yield |
|---|---|
| (1,2-bis(diphenylphosphino)ethane)palladium(II) chloride In 1,4-dioxane for 1.5h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In 1,4-dioxane for 1h; Heating; | 100% |
-
-
99277-71-1
4-bromo-2-nitrobenzoic acid

-
-
544-97-8
dimethyl zinc(II)

-
-
27329-27-7
4-methyl-2-nitrobenzoic acid

| Conditions | Yield |
|---|---|
| (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In 1,4-dioxane for 2h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In 1,4-dioxane for 1h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: carbon dioxide; dimethyl zinc(II); 1-methoxy-4-(4-phenylbut-1-yn-1-yl)benzene With bis(1,5-cyclooctadiene)nickel (0); 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran; hexane at 20℃; Stage #2: diazomethane In diethyl ether at 0℃; | 100% |

-
-
770711-59-6
(3Z,5R,6R,7E,9S)-1-trimethylsilyl-3-bromo-5,7,9-trimethyl-6-(t-butyldimethylsilyloxy)-11-phenyl-3,7-undecadien-1-yne

-
-
544-97-8
dimethyl zinc(II)

-
-
770711-60-9
(3E,5R,6R,7E,9S)-1-trimethylsilyl-3,5,7,9-tetramethyl-6-(t-butyldimethylsilyloxy)-11-phenyl-3,7-undecadien-1-yne

| Conditions | Yield |
|---|---|
| With bis(tri-t-butylphosphine)palladium(0) In tetrahydrofuran; toluene at 23℃; for 1h; | 100% |

-
-
544-97-8
dimethyl zinc(II)

| Conditions | Yield |
|---|---|
| Stage #1: C18H17F4NO3S; dimethyl zinc(II); (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride In 1,4-dioxane; toluene for 2h; Heating / reflux; Stage #2: With methanol; water In 1,4-dioxane; ethyl acetate; toluene | 100% |

-
-
544-97-8
dimethyl zinc(II)

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; diethyl ether stirring (room temp., 0.5 h); evapn., chromy. (petroleum ether / CH2Cl2 = 3 : 1); elem. anal.; | 100% |
-
-
868840-71-5
(HOC6H2(C(CH3)3)CH2)2(C6H2(C(CH3)3)(CCC6H5)CH2)2

-
-
544-97-8
dimethyl zinc(II)

-
-
947622-65-3
(CH3)2Zn2((OC6H2(C(CH3)3)CH2)2(C6H2(C(CH3)3)(CCC6H5)CH2)2)

| Conditions | Yield |
|---|---|
| In toluene under N2; soln. of ZnMe2 in toluene added to soln. of ligand in toluene;stirred at room temp. for 8 h; volatiles evapd.; | 100% |
-
-
947622-63-1
(HOC6H2(C(CH3)3)CH2)2(C6H2(C(CH3)3)(CCC6H4CH3)CH2)2

-
-
544-97-8
dimethyl zinc(II)

-
-
947622-67-5
(CH3)2Zn2((OC6H2(C(CH3)3)CH2)2(C6H2(C(CH3)3)(CCC6H4CH3)CH2)2)

| Conditions | Yield |
|---|---|
| In toluene under N2; soln. of ZnMe2 in toluene added to soln. of ligand in toluene;stirred at room temp. for 8 h; volatiles evapd.; elem. anal.; | 100% |
-
-
947622-62-0
(HOC6H2(C(CH3)3)CH2)2(C6H2(C(CH3)3)(CCC6H4OCH3)CH2)2

-
-
544-97-8
dimethyl zinc(II)

-
-
947622-66-4
(CH3)2Zn2((OC6H2(C(CH3)3)CH2)2(C6H2(C(CH3)3)(CCC6H4OCH3)CH2)2)

| Conditions | Yield |
|---|---|
| In toluene under N2; soln. of ZnMe2 in toluene added to soln. of ligand in toluene;stirred at room temp. for 8 h; volatiles evapd.; elem. anal.; | 100% |
-
-
943231-28-5
5,11,17,23-tetra(tert-butyl)-25,27-dihydroxy-26,28-bis[p-(trifluoromethyl)phenylethynyl]calix[4]arene

-
-
544-97-8
dimethyl zinc(II)

-
-
947622-68-6
(CH3)2Zn2((OC6H2(C(CH3)3)CH2)2(C6H2(C(CH3)3)(CCC6H4CF3)CH2)2)

| Conditions | Yield |
|---|---|
| In toluene under N2; soln. of ZnMe2 in toluene added to soln. of ligand in toluene;stirred at room temp. for 8 h; volatiles evapd.; elem. anal.; | 100% |
-
-
947622-79-9
(HOC6H2(C(CH3)3)CH2)2(C6H2(C(CH3)3)(CCC6H4CF3)CH2)C6H2(C(CH3)3)(CCC6H4OCH3)CH2

-
-
544-97-8
dimethyl zinc(II)

-
-
947622-80-2
(CH3)2Zn2((OC6H2(C(CH3)3)CH2)2(C6H2(C(CH3)3)(CCC6H4CF3)CH2)C6H2(C(CH3)3)(CCC6H4OCH3)CH2)

| Conditions | Yield |
|---|---|
| In toluene under N2; soln. of ZnMe2 in toluene added to soln. of ligand in toluene;stirred at room temp. for 8 h; volatiles evapd.; elem. anal.; | 100% |
-
-
947622-64-2
(HOC6H2(C(CH3)3)CH2)2(C6H2(C(CH3)3)(CCC6H3(CF3)2)CH2)2

-
-
544-97-8
dimethyl zinc(II)

-
-
947622-69-7
(CH3)2Zn2((OC6H2(C(CH3)3)CH2)2(C6H2(C(CH3)3)(CCC6H3(CF3)2)CH2)2)

| Conditions | Yield |
|---|---|
| In toluene under N2; soln. of ZnMe2 in toluene added to soln. of ligand in toluene;stirred at room temp. for 8 h; volatiles evapd.; | 100% |
-
-
939994-88-4
(3aR,4E,7aS)-4-(bromomethylene)-3a,4,5,6,7,7a-hexahydro-7a-methyl-3H-indene-1-carbaldehyde

-
-
544-97-8
dimethyl zinc(II)

-
-
939994-50-0
(S)-1-{(3aS,7E,7aR)-7-(bromomethylene)-3a,4,5,6,7,7a-hexahydro-3a-methyl-1H-indene-3-yl}ethanol

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate; N,N’-(1R,2R)-cyclohexane-1,2-diylbis(1,1,1-trifluoromethanesulfonamide) In tert-butyl methyl ether at 20℃; for 1h; Product distribution / selectivity; | 100% |
-
-
3839-31-4
dimethyl(phenyl)silyl lithium

-
-
583-55-1
1-Bromo-2-iodobenzene

-
-
544-97-8
dimethyl zinc(II)

-
-
778-24-5
Dimethyldiphenylsilane

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl(phenyl)silyl lithium; dimethyl zinc(II) In tetrahydrofuran; hexane at 0℃; for 0.5h; Stage #2: 1-Bromo-2-iodobenzene In tetrahydrofuran; hexane at 20℃; for 16h; Further stages.; | 100% |


| Conditions | Yield |
|---|---|
| With zinc trifluoromethanesulfonate In hexane; dichloromethane at 0 - 20℃; for 1h; | 100% |
| With zinc trifluoromethanesulfonate In hexane; dichloromethane at 0 - 20℃; for 1h; | 100% |
| With zinc trifluoromethanesulfonate In hexane; dichloromethane at 0 - 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride In tetrahydrofuran at 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; diisobutylaluminium hydride In 1,4-dioxane at 100℃; for 2.5h; | 100% |
-
-
1210419-46-7
methyl 6-(2,6-difluoro-3-(trifluoromethylsulfonyloxy)-phenyl)picolinate

-
-
544-97-8
dimethyl zinc(II)

-
-
1210419-50-3
methyl 6-(2,6-difluoro-3-methylphenyl)picolinate

| Conditions | Yield |
|---|---|
| dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 In toluene at 80℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In hexane at 0 - 20℃; for 12h; Inert atmosphere; | 100% |
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran; hexane at 0 - 20℃; for 12h; | 69.5 mg |
-
-
1291092-82-4
Re(κ1(P)-6-(diphenylphosphino)-2,2'-bipyridine)(CO)5][OTf]

-
-
544-97-8
dimethyl zinc(II)

-
-
1291092-89-1
[Re(κ1(P)-6-(diphenylphosphino)-2,2'-bipyridine*ZnMe)(μ2-COMe)(CO)4][OTf]

| Conditions | Yield |
|---|---|
| In dichloromethane-d2 Zn compd. added to a soln. of Re complex, shaken; evapd. (vac.), triturated with pentane; obtained as an oil; elem. anal.; | 100% |
-
-
16184-89-7
4'-bromo-2,2,2-trifluoroacetophenone

-
-
544-97-8
dimethyl zinc(II)

-
-
122243-28-1
2‐(4‐bromophenyl)‐1,1,1‐trifluoropropan‐2‐ol

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In n-heptane; dichloromethane at -40 - 20℃; for 2.08333h; | 100% |
| Stage #1: dimethyl zinc(II) With titanium tetrachloride In n-heptane; dichloromethane at -30℃; for 0.5h; Stage #2: 4'-bromo-2,2,2-trifluoroacetophenone In n-heptane; dichloromethane at -40 - 20℃; for 2h; | 100% |
| Stage #1: dimethyl zinc(II) With titanium tetrachloride In n-heptane; dichloromethane at -30℃; for 0.5h; Stage #2: 4'-bromo-2,2,2-trifluoroacetophenone In n-heptane; dichloromethane at -40 - 20℃; for 2.08333h; | 100% |

-
-
544-97-8
dimethyl zinc(II)

| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate In toluene at 20℃; Inert atmosphere; | 100% |

-
-
544-97-8
dimethyl zinc(II)

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0) In tetrahydrofuran; toluene at 0℃; for 0.166667h; Negishi Coupling; Inert atmosphere; | 100% |
-
-
544-97-8
dimethyl zinc(II)

| Conditions | Yield |
|---|---|
| With titanium tetrachloride In dichloromethane; toluene at -40 - 20℃; Inert atmosphere; | 100% |
-
-
544-97-8
dimethyl zinc(II)

| Conditions | Yield |
|---|---|
| Stage #1: dimethyl zinc(II) With titanium tetrachloride In toluene at -40℃; for 0.5h; Inert atmosphere; Stage #2: 1-bromo-3,6-dimethyl-9H-fluoren-9-one In dichloromethane; toluene at 20℃; Inert atmosphere; | 100% |
Dimethylzinc History
This substance was first prepared by Edward Frankland during his work with Robert Bunsen in 1849 at the University of Marburg. After heating a mixture of zinc and methyl iodide in an airtight vessel a flame bursted out after the seal was broken In laboratory scale the synthesis method did not change till today, except that copper or copper compounds are used to activate the zinc.
Dimethyl zinc was used for a long time to introduce methyl groups into organic molecules or to synthesis organometalic compounds containing methyl groups. The Grignard reagents, a magnesium organic compound, which is easier to handle and less imflamable substituted zinc methyl in most of the laboratory synthesis. There are, however, several reactions which proceed better or result in alternative products with the zinc organic compounds than with magnesium organic compounds.
Dimethylzinc Consensus Reports
Zinc and its compounds are on the Community Right-To-Know List.
Dimethylzinc Specification
The Zinc, dimethyl-, with the CAS registry number 544-97-8, is also known as Methylzinc. It belongs to the product categories of Dialkylzincs; Organometallic Reagents; Reike and Organozinc Reagents. Its EINECS number is 208-884-1. This chemical's molecular formula is C2H6Zn and molecular weight is 95.46. What's more, its systematic name is dimethylzinc. It is air and moisture sensitive. It has been of great importance in the synthesis of organic compounds.
Preparation: this chemical can be prepared by the action of methyl iodide on a zinc at elevated temperature or on zinc sodium alloy.
3Zn + 2CH3I → Zn(CH3)2 + ZnI2
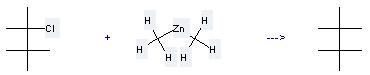
Uses of Zinc, dimethyl-: it can be used to produce 2,2,3,3-tetramethyl-butane at the temperature of -50 - -20 °C. It will need reagent ZnCl2 and solvent CH2Cl2. The yield is about 80%.
When you are using this chemical, please be cautious about it as the following:
This chemical is highly flammable and is spontaneously flammable in air, so you should keep it away from sources of ignition - No smoking. It will react violently with water and liberate extremely flammable gases. It can cause burns. It has possible risk of harm to the unborn child. This chemical is harmful as it may cause lung damage if swallowed and has the danger of serious damage to health by prolonged exposure through inhalation. Its vapours may cause drowsiness and dizziness. It is toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. You should not empty it into drains. You must take precautionary measures against static discharges. You must avoid releasing it to the environment just refering to special instructions/safety data sheet. If swallowed, it will not induce vomiting but you need seek medical advice immediately and show this container or label to the doctor. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need wear suitable protective clothing, gloves and eye/face protection. This material and its container must be disposed of as hazardous waste. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: C[Zn]C
(2)Std. InChI: InChI=1S/2CH3.Zn/h2*1H3;
(3)Std. InChIKey: AXAZMDOAUQTMOW-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View