-
Name
Diphenylacetonitrile
- EINECS 201-662-5
- CAS No. 86-29-3
- Article Data132
- CAS DataBase
- Density 1.076 g/cm3
- Solubility Soluble in alcohol, ether
- Melting Point 71-73 °C(lit.)
- Formula C14H11N
- Boiling Point 322.3 °C at 760 mmHg
- Molecular Weight 193.248
- Flash Point 151.6 °C
- Transport Information
- Appearance white to creamy or faint yellow crystalline powder
- Safety 26-36-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Diphenyl acetonitriie;Diphenyl-alpha-cyanomethane;Diphenylmethyl-cyanide;Diphenyl acetonitrile;α-Phenylbenzylcyanide;α-phenyl-phenylacetonitrile;Diphenyl-α-cyanomethane;α-Cyanodiphenylmethane;alpha-Phenylbenzeneacetonitrile;Diphenyl-.alpha.-cyanomethane;Benzeneacetonitrile,R-phenyl-;Diphenatrile;alpha-Cyanodiphenylmethane;alpha-Phenylbenzylcyanide;Benzeneacetonitrile, .alpha.-phenyl-;Acetonitrile, diphenyl-;alpha-Phenylphenylacetonitrile;2,2-diphenylacetonitrile;
- PSA 23.79000
- LogP 3.34208
Synthetic route
-
-
15795-74-1
1,1-diphenyl-2,2-dinitroethylene

-
-
86-29-3
Diphenylacetonitrile

| Conditions | Yield |
|---|---|
| With samarium diiodide In tetrahydrofuran for 2h; Ambient temperature; | 100% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
5433-78-3
diphenylmethyl p-tolyl sulfone

-
-
86-29-3
Diphenylacetonitrile

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride In dichloromethane at 25℃; for 1h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With copper diacetate; 1,2-bis-(dicyclohexylphosphino)ethane In tetrahydrofuran at 20℃; for 12h; Catalytic behavior; Mechanism; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; | 99% |
| With bis(trichloromethyl) carbonate; triethylamine In chloroform at 50℃; for 2h; | 87% |
| With palladium diacetate; Selectfluor; acetonitrile at 20℃; for 18h; | 84% |

| Conditions | Yield |
|---|---|
| With iodine; lithium carbonate In dichloromethane at 35℃; for 5h; | 99% |
| With titanium tetrachloride In dichloromethane at 0℃; for 2h; | 93% |
-
-
41401-03-0
diphenyl-acetaldehyde-oxime

-
-
86-29-3
Diphenylacetonitrile

| Conditions | Yield |
|---|---|
| With trifluoromethylsulfonic anhydride; Triphenylphosphine oxide In dichloromethane; 1,2-dichloro-ethane for 0.0833333h; Ambient temperature; | 98% |
| With 4-nitro-1-((trifluoromethyl)sulfonyl)-1H-imidazole; triethylamine In acetonitrile at 20℃; for 0.166667h; Inert atmosphere; Sealed tube; | 78% |

| Conditions | Yield |
|---|---|
| IrH5(P-(i-Pr)3)2 In toluene at 150℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| With iodine; lithium carbonate In dichloromethane at 35℃; for 5h; | 98% |
| With tris(pentafluorophenyl)borate In acetonitrile at 20℃; for 1h; | 93% |
| With zinc trifluoromethanesulfonate In nitromethane at 100℃; for 5.5h; | 90% |
| With 3-dodecyl-2-iodo-1-methyl-1H-imidazol-3-ium hexafluoroantimonate; iodine In nitromethane at 20℃; for 2h; Inert atmosphere; | 46% |

| Conditions | Yield |
|---|---|
| With gold(III) chloride In dichloromethane at 20℃; for 0.0833333h; Reagent/catalyst; Time; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With P(i-BuNCH2CH2)3N; sodium hexamethyldisilazane; palladium diacetate In toluene at 100℃; for 6h; | 93% |
| With potassium phosphate; dicyclohexyl-({2-[2-(dicyclohexylphosphanyl)phenyl]-phenyl})-phosphane; palladium diacetate In 1,4-dioxane at 80℃; for 24h; Inert atmosphere; Sealed tube; | 89% |
| With potassium hydroxide In water at 45℃; for 17h; | 84% |
| With caesium carbonate; triphenylphosphine; palladium dichloride In N,N-dimethyl-formamide at 130℃; for 2h; Phenylation; | 44% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid; trifluoroacetic anhydride; sodium nitrite at 20℃; nitrosative cleavage; | 93% |

| Conditions | Yield |
|---|---|
| With ammonia; oxygen In tert-Amyl alcohol at 40℃; under 750.075 Torr; for 24h; Green chemistry; | 93% |
| With HCl·DMPU; hydroxylamine hydrochloride In acetonitrile at 60℃; | 92% |
| Multi-step reaction with 3 steps 1: ammonia / water / 20 °C / Green chemistry 2: iodine / water / 20 °C / Green chemistry 3: ammonia / water / 20 °C / Green chemistry View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: phenylacetonitrile With tris-(dibenzylideneacetone)dipalladium(0); bis(proazaphosphatrane); potassium tert-butylate In toluene at 20℃; for 0.333333h; Schlenk technique; Inert atmosphere; Stage #2: chlorobenzene In toluene at 80℃; for 4h; Inert atmosphere; Schlenk technique; | 92% |
| With sodium hexamethyldisilazane; 2,8,9-tris(2-methylpropyl)-2,5,8,9-tetraaza-1-phosphabicyclo[3.3.3]undecane; palladium diacetate In toluene at 90℃; for 6h; | 91% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; 18-crown-6 ether In acetonitrile for 6h; Heating; | 91% |
-
-
10442-39-4
tetra-n-butylammonium cyanide

-
-
79373-25-4
2-(diphenylmethyloxy)tetrahydro-2H-pyran

-
-
86-29-3
Diphenylacetonitrile

| Conditions | Yield |
|---|---|
| With triphenylphosphine; 2,3-dicyano-5,6-dichloro-p-benzoquinone In acetonitrile for 6.5h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With sodium azide; TEA; triethylphosphine; (bis-(2-methoxyethyl)amino)sulfur trufluoride In dichloromethane; dimethyl sulfoxide at 0℃; for 30h; | 90% |
| With sodium azide; triethylamine; triphenylphosphine; (bis-(2-methoxyethyl)amino)sulfur trufluoride In dichloromethane; dimethyl sulfoxide at 0 - 20℃; for 0.833333h; | 88% |
| Multi-step reaction with 2 steps 1: diethyl ether; NH3 / 230 °C / im geschlossenen Rohr 2: PCl5; POCl3 View Scheme | |
| Multi-step reaction with 3 steps 1: thionyl chloride 2: ammonia 3: thionyl chloride / 90 - 105 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: thionyl chloride / tetrahydrofuran / 1 h / 50 °C 1.2: 0.08 h / 0 °C 2.1: palladium diacetate; Selectfluor; acetonitrile / 18 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: benzyl alcohol With sodium methylate In ethyl acetate; acetonitrile at 70℃; for 2h; Industrial scale; Stage #2: phenylacetonitrile at 110℃; for 10h; Concentration; Industrial scale; | 90% |
-
-
85823-49-0
Diphenyl-acetonitrile N-oxide

-
-
86-29-3
Diphenylacetonitrile

| Conditions | Yield |
|---|---|
| With acetic acid; zinc In methanol for 3h; Ambient temperature; | 89.3% |

| Conditions | Yield |
|---|---|
| With iron(III) trifluoromethanesulfonate In 1,2-dichloro-ethane at 50℃; for 1h; | 88% |
-
-
640-60-8
toluene-4-sulfonic acid phenyl ester

-
-
140-29-4
phenylacetonitrile

-
-
86-29-3
Diphenylacetonitrile

| Conditions | Yield |
|---|---|
| With potassium phosphate; palladium diacetate; XPhos In dichloromethane; tert-butyl alcohol at 110℃; for 4h; Inert atmosphere; Schlenk technique; Sealed tube; | 87% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; potassium carbonate; XPhos In dichloromethane; tert-butyl alcohol at 110℃; for 4h; Inert atmosphere; Schlenk technique; Sealed tube; | 86% |

| Conditions | Yield |
|---|---|
| With trimethylsilyl cyanide; zinc trifluoromethanesulfonate at 100℃; for 6.5h; Time; Inert atmosphere; Schlenk technique; | 85% |

| Conditions | Yield |
|---|---|
| With iodine; lithium carbonate In dichloromethane at 35℃; for 5h; | 85% |
-
-
108-86-1
bromobenzene

-
-
140-29-4
phenylacetonitrile

-
A
-
86-29-3
Diphenylacetonitrile

-
B
-
6639-43-6
2,2,2-triphenylacetonitrile

| Conditions | Yield |
|---|---|
| With ethoxyethoxyethanol; sodium amide In 1,2-dimethoxyethane 1.) 45 deg C, 2 h, 2.) 0 deg C, 0.5 h, 3.) 0 deg C, 2 h; | A 84% B 15% |
| With potassium phosphate; tri-tert-butylphosphonium tetrafluoroborate; palladium diacetate In 1,4-dioxane; water at 100℃; for 12h; Inert atmosphere; Sealed tube; | A 33% B 45% |


| Conditions | Yield |
|---|---|
| With P(i-BuNCH2CH2)3N; sodium hexamethyldisilazane; palladium diacetate In toluene at 100℃; for 16h; | 81% |
| Stage #1: acetonitrile With sodium hexamethyldisilazane In toluene at 25℃; for 0.166667h; Stage #2: bromobenzene With 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; palladium diacetate In toluene at 100℃; for 16h; Further stages.; | 68.4% |
-
-
12082-05-2
(η6-fluorobenzene)tricarbonylchromium

-
-
140-29-4
phenylacetonitrile

-
-
86-29-3
Diphenylacetonitrile

| Conditions | Yield |
|---|---|
| With potassium hydroxide; iodine In diethyl ether; dimethyl sulfoxide addn. of C6H5CH2CN to mixt. of Cr(CO)3-complex/powd. KOH in DMSO (stirring; 20°C, 1 h), diln. with aq. HCl, extn. with Et2O, treating with excess I2 (room temp., 3 h); distn. (reduced pressure); identification by GC and NMR; | 80% |
-
-
108-86-1
bromobenzene

-
-
75-05-8
acetonitrile

-
A
-
86-29-3
Diphenylacetonitrile

-
B
-
140-29-4
phenylacetonitrile

| Conditions | Yield |
|---|---|
| With ethoxyethoxyethanol; sodium amide In 1,2-dimethoxyethane 1.) 45 deg C, 2 h, 2.) -10 deg C, 2 h, 3.) -30 deg C, 14 h; | A 17% B 79% |


| Conditions | Yield |
|---|---|
| With sulfuric acid at 40 - 45℃; for 1h; | 78% |
| With aluminium trichloride anschliessendes Erwaermen; | |
| With phosphorus pentoxide |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 80℃; for 12h; | 75% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In trifluoroacetic acid for 6h; Ambient temperature; | 71% |

| Conditions | Yield |
|---|---|
| With methanol; sodium bromide In tetrahydrofuran at 20℃; for 0.7h; Electrolysis; | 100% |
| With 2-chloro-2-methyl-3-(p-nitrophenyl)-3-propanone; potassium tert-butylate In dimethyl sulfoxide at 20℃; for 1.5h; | 86% |
| With 2,6-diphenyl-4-methoxyphenoxyl radical In chlorobenzene at 110℃; for 0.75h; | 65% |
-
-
50-00-0
formaldehyd

-
-
86-29-3
Diphenylacetonitrile

-
-
92552-36-8
α-hydroxymethyl-α-phenylbenzenacetonitrile

| Conditions | Yield |
|---|---|
| With pyridine; N-benzyl-trimethylammonium hydroxide In water at 0 - 23℃; | 100% |
| With N-benzyl-trimethylammonium hydroxide In methanol; toluene at 20℃; for 2h; | 98% |
| With water; calcium oxide In tetrahydrofuran at 30℃; |

| Conditions | Yield |
|---|---|
| Stage #1: Diphenylacetonitrile With lithium hexamethyldisilazane In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: allyl bromide In tetrahydrofuran at -78 - 20℃; Inert atmosphere; | 100% |
| Stage #1: Diphenylacetonitrile With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at 0℃; for 1h; Stage #2: allyl bromide In tetrahydrofuran; hexane at 20℃; for 3h; | 98% |
| Stage #1: Diphenylacetonitrile With sodium hydride In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: allyl bromide In N,N-dimethyl-formamide at 0 - 20℃; | 93% |
-
-
86-29-3
Diphenylacetonitrile

-
-
26055-95-8
3(S)-(4-methylphenylsulfonyloxy)-1-phenylmethyl pyrrolidine

-
-
197964-14-0
2,2-diphenyl-2-[(3S)-N-benzyl-pyrrolidin-3-yl]acetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: Diphenylacetonitrile With sodium hydride In toluene for 2h; Reflux; Stage #2: 3(S)-(4-methylphenylsulfonyloxy)-1-phenylmethyl pyrrolidine In toluene for 3h; Reflux; | 100% |
-
-
6737-11-7
2-acetoxy-3-butene

-
-
86-29-3
Diphenylacetonitrile

-
-
60171-90-6
3-methyl-2,2-diphenylpent-4-enenitrile

| Conditions | Yield |
|---|---|
| Stage #1: Diphenylacetonitrile With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 1h; Inert atmosphere; Schlenk technique; Stage #2: 2-acetoxy-3-butene With bis[(1,2,3-η)-2-butenyl]di(μ-chloro)palladium(II); tri-tert-butyl phosphine In tetrahydrofuran; mineral oil at 0 - 20℃; for 16h; Tsuji-Trost Allylation; Inert atmosphere; Schlenk technique; regioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; N-benzyl-N,N,N-triethylammonium chloride In toluene for 3h; | 99% |
| With tetrabutylammomium bromide; oxygen In N,N-dimethyl-formamide Hg cathode, Pt anode, -1.0 V vs SCE; | 95% |
| With oxygen; potassium carbonate In water; dimethyl sulfoxide Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran; mineral oil at 85℃; for 2h; Inert atmosphere; Sealed tube; | 99% |
| With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 2h; Inert atmosphere; | 92% |
| With sodium amide 1.) benzene, reflux, 2 h, 2.) benzene, reflux, 2 h; Yield given. Multistep reaction; | |
| Stage #1: Diphenylacetonitrile With sodium hydride In tetrahydrofuran at 0 - 20℃; for 1h; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran at 0 - 20℃; for 13h; Inert atmosphere; | |
| Stage #1: Diphenylacetonitrile With sodium hexamethyldisilazane In tetrahydrofuran at -78℃; for 0.5h; Stage #2: methyl iodide In tetrahydrofuran at -78 - 20℃; |
-
-
86-29-3
Diphenylacetonitrile

-
-
116183-79-0
(3S)-3-<(4-tolylsulfonyl)oxy>-1-(phenylmethyl)pyrrolidine

-
-
197964-14-0
2,2-diphenyl-2-[(3S)-N-benzyl-pyrrolidin-3-yl]acetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: Diphenylacetonitrile With potassium tert-butylate In tetrahydrofuran at 0℃; for 1.08333h; Stage #2: (3S)-3-<(4-tolylsulfonyl)oxy>-1-(phenylmethyl)pyrrolidine In tetrahydrofuran at 0 - 40℃; | 99% |
| With potassium tert-butylate In tetrahydrofuran at 0 - 40℃; for 16 - 26h; | 99% |
| Stage #1: Diphenylacetonitrile With potassium tert-butylate In tetrahydrofuran at 0℃; for 1.08333h; Stage #2: (3S)-3-<(4-tolylsulfonyl)oxy>-1-(phenylmethyl)pyrrolidine In tetrahydrofuran at 0 - 40℃; for 15.0833 - 25.1667h; | 99% |
| Stage #1: Diphenylacetonitrile With potassium tert-butylate In tetrahydrofuran at 0℃; for 1.08333h; Stage #2: (3S)-3-<(4-tolylsulfonyl)oxy>-1-(phenylmethyl)pyrrolidine In tetrahydrofuran at 0 - 40℃; for 15.0833 - 25.1667h; | 99% |
| Stage #1: Diphenylacetonitrile With potassium tert-butylate In tetrahydrofuran at 0℃; for 1.08333h; Stage #2: (3S)-3-<(4-tolylsulfonyl)oxy>-1-(phenylmethyl)pyrrolidine In tetrahydrofuran at 0 - 40℃; for 15 - 25h; | 99% |

-
-
86-29-3
Diphenylacetonitrile

| Conditions | Yield |
|---|---|
| In hexane at ambient temp.; XRD; | 99% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran; mineral oil at 85℃; for 2h; Inert atmosphere; Sealed tube; | 99% |
| Stage #1: Diphenylacetonitrile With lithium dimethylamide In tetrahydrofuran at -78℃; for 0.5h; Inert atmosphere; Stage #2: bromopentene In tetrahydrofuran at 20℃; Inert atmosphere; | |
| Stage #1: Diphenylacetonitrile With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; Inert atmosphere; Stage #2: bromopentene In N,N-dimethyl-formamide; mineral oil at 0 - 20℃; for 12h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); carbon dioxide In dimethyl sulfoxide at 80℃; under 760.051 Torr; for 14h; Reagent/catalyst; Sealed tube; | 99% |
| With potassium carbonate In toluene at 80℃; for 18h; Catalytic behavior; Green chemistry; | 94% |
| With 1,1'-bis-(diphenylphosphino)ferrocene; bis(1,5-cyclooctadiene)nickel (0) In methanol at 80℃; Sealed tube; Schlenk technique; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: Diphenylacetonitrile With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 1h; Stage #2: 1-Bromo-2-butyne In tetrahydrofuran; toluene; mineral oil at 20℃; | 99% |
| Stage #1: Diphenylacetonitrile With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 2h; Stage #2: 1-Bromo-2-butyne In tetrahydrofuran at -78 - 0℃; |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 2-(dicyclohexylphosphino)-2'-methylbiphenyl In N,N-dimethyl-formamide at 80℃; for 1h; Inert atmosphere; Glovebox; Sealed tube; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 2-(dicyclohexylphosphino)-2'-methylbiphenyl In N,N-dimethyl-formamide at 80℃; for 1h; Inert atmosphere; Glovebox; Sealed tube; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 2-(dicyclohexylphosphino)-2'-methylbiphenyl In N,N-dimethyl-formamide at 80℃; for 1h; Inert atmosphere; Glovebox; Sealed tube; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 2-(dicyclohexylphosphino)-2'-methylbiphenyl In N,N-dimethyl-formamide at 80℃; for 1h; Inert atmosphere; Glovebox; Sealed tube; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With triethylamine alane In tetrahydrofuran for 1h; Ambient temperature; | 98.5% |
| With ammonia; hydrogen In toluene at 120℃; under 22502.3 Torr; for 16h; Autoclave; | 90% |
| With lithium aluminium tetrahydride; diethyl ether |

| Conditions | Yield |
|---|---|
| With PEG-400; sodium hydroxide for 0.0333333h; microwave irradiation; | 98% |
| With potassium hydroxide at 150℃; for 2h; | 80% |
| With sodium azide; acetic acid In 1-methyl-pyrrolidin-2-one; water at 220℃; for 0.266667h; | 73% |
| Multi-step reaction with 2 steps 1: sodium azide; acetic acid / 1-methyl-pyrrolidin-2-one; water / 0.27 h / 220 °C / Microwave irradiation 2: acetic acid / 1-methyl-pyrrolidin-2-one; water / 240 °C View Scheme |
-
-
86-29-3
Diphenylacetonitrile

-
-
23326-27-4
Methyl 2-butynoate

-
-
946617-85-2
(E)-methyl 4-cyano-3-methyl-4,4-diphenylbut-2-enoate

| Conditions | Yield |
|---|---|
| With N-benzyl-trimethylammonium hydroxide In 1,4-dioxane; methanol for 2h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: Diphenylacetonitrile With sodium hydride In tetrahydrofuran at 20℃; for 1h; Stage #2: propargyl bromide In tetrahydrofuran at 20℃; for 12h; | 97% |
| Stage #1: Diphenylacetonitrile With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 1h; Stage #2: propargyl bromide In tetrahydrofuran; toluene; mineral oil at 20℃; | 94% |
| Stage #1: Diphenylacetonitrile With sodium hydride In tetrahydrofuran; mineral oil at 0 - 20℃; for 1h; Stage #2: propargyl bromide In tetrahydrofuran; toluene; mineral oil | 94% |

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran; mineral oil at 85℃; for 2h; Inert atmosphere; Sealed tube; | 97% |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 2-(dicyclohexylphosphino)-2'-methylbiphenyl In N,N-dimethyl-formamide at 80℃; for 1h; Inert atmosphere; Glovebox; Sealed tube; regioselective reaction; | 97% |
-
-
86-29-3
Diphenylacetonitrile

-
-
40400-13-3
2-Iodobenzyl bromide

| Conditions | Yield |
|---|---|
| Stage #1: Diphenylacetonitrile With sodium hydride In N,N-dimethyl-formamide; benzene at 0℃; for 0.5h; Stage #2: 2-Iodobenzyl bromide In N,N-dimethyl-formamide; benzene at 0 - 20℃; Further stages.; | 96% |
Diphenylacetonitrile Consensus Reports
Diphenylacetonitrile Specification
The Diphenylacetonitrile with CAS registry number of 86-29-3 is also known as Diphenyl-alpha-cyanomethane. The IUPAC name is 2,2-Diphenylacetonitrile. It belongs to product categories of Pharmaceutical Intermediates; Aromatic Nitriles; C10 to C27; Cyanides/Nitriles; Nitrogen Compounds. Its EINECS registry number is 201-662-5. In addition, the formula is C14H11N and the molecular weight is 193.24. This chemical is a white crystal.
Physical properties about Diphenylacetonitrile are: (1)ACD/LogP: 3.30; (2)ACD/LogD (pH 5.5): 3.3; (3)ACD/LogD (pH 7.4): 3.3; (4)ACD/BCF (pH 5.5): 189.12; (5)ACD/BCF (pH 7.4): 189.12; (6)ACD/KOC (pH 5.5): 1483.54; (7)ACD/KOC (pH 7.4): 1483.54; (8)#H bond acceptors: 1; (9)#Freely Rotating Bonds: 2; (10)Index of Refraction: 1.584; (11)Molar Refractivity: 60.11 cm3; (12)Molar Volume: 179.4 cm3; (13)Surface Tension: 45 dyne/cm; (14)Density: 1.076 g/cm3; (15)Flash Point: 151.6 °C; (16)Enthalpy of Vaporization: 56.41 kJ/mol; (17)Boiling Point: 322.3 °C at 760 mmHg; (18)Vapour Pressure: 0.000282 mmHg at 25 °C.
Preparation of Diphenylacetonitrile: it is prepared by reaction of 2,2-diphenyl-acetamide. The reaction needs reagents bis(trichloromethyl) carbonate, triethylamine and solvent CHCl3 at the temperature of 50 °C for 2 hours. The yield is about 87%.

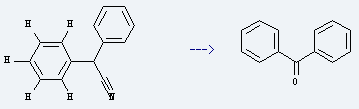
Uses of Diphenylacetonitrile: it is used to produce benzophenone. The reaction occurs with reagents O2, K2CO3 and solvents dimethylsulfoxide, H2O at ambient temperature. The yield is about 95%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing, gloves and eye/face protection. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=C(C=C1)C(C#N)C2=CC=CC=C2
2. InChI: InChI=1S/C14H11N/c15-11-14(12-7-3-1-4-8-12)13-9-5-2-6-10-13/h1-10,14H
3. InChIKey: NEBPTMCRLHKPOB-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LD50 | unreported | 3500mg/kg (3500mg/kg) | "Chemistry of Pesticides," Melnikov, N.N., New York, Springer-Verlag New York, Inc., 1971Vol. -, Pg. 155, 1971. | |
| mouse | LD50 | intraperitoneal | 200mg/kg (200mg/kg) | National Technical Information Service. Vol. AD691-490, | |
| mouse | LD50 | intravenous | 100mg/kg (100mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#04134, | |
| rat | LD50 | oral | 3500mg/kg (3500mg/kg) | Residue Reviews. Vol. 10, Pg. 97, 1965. |
Related Products
- Diphenylacetonitrile
- 86293-48-3
- 86293-52-9
- 86299-46-9
- 86299-47-0
- 862999-75-5
- 862999-80-2
- 86303-22-2
- 86303-23-3
- 86-30-6
- 863127-76-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View