-
Name
5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one
- EINECS 201-891-0
- CAS No. 89-25-8
- Article Data198
- CAS DataBase
- Density 1.17 g/cm3
- Solubility 3 g/L (20 °C) in water
- Melting Point 126-128 °C(lit.)
- Formula C10H10N2O
- Boiling Point 333 °C at 760 mmHg
- Molecular Weight 174.202
- Flash Point 155.2 °C
- Transport Information
- Appearance Off white to light yellow powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms C.I. Developer 1;Radicut;CI Developer 1;1-Phenyl-3-methylpyrazolone-5;Methylphenylpyrazolone;3H-Pyrazol-3-one, 2, 4-dihydro-5-methyl-2-phenyl-;2-Pyrazolin-5-one, 3-methyl-1-phenyl-;Edaravone (JAN);3H-Pyrazol-3-one, 2,4-dihydro-5-methyl-2-phenyl-;Norphenazone;1-Phenyl-3-methylpyrazolone;1-Phenyl-3-methyl-5-pyrazolone;Developer Z;1-Phenyl-3-methyl-5-oxo-2-pyrazoline;Jarocol PMP;1-Phenyl-3-Methyl-5-Pyrazolone(PMP);1Phenyl 3Methyl 5Pyrazolone;3-methyl-1-phenyl-1H-pyrazol-5(4H)-one;
- PSA 32.67000
- LogP 1.29980
Synthetic route

| Conditions | Yield |
|---|---|
| In acetic acid Heating; | 100% |
| for 0.166667h; Irradiation; | 100% |
| at 0 - 90℃; for 1.5h; | 100% |

| Conditions | Yield |
|---|---|
| With acetic acid for 3h; Reflux; | 98% |
| With cellulose sulfuric acid In water at 20℃; for 0.133333h; Knorr Pyrazole Synthesis; Green chemistry; regioselective reaction; | 92% |
| With Fe3O4(at)SiO2-bonded N-propyl-diethylenetetrasulfamic acid In water at 20℃; for 0.416667h; Catalytic behavior; Green chemistry; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-methyleneoxetan-2-one With ammonium hydroxide at 10℃; for 0.5h; Stage #2: phenylhydrazine at 60℃; for 2h; Stage #3: With hydrogenchloride In water for 1h; pH=5; | 93% |
-
-
13292-56-3
5-methyl-2-phenylpyrazolidin-3-one

-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With sodium anthraquinone-2-sulfonate; palladium diacetate; potassium carbonate In chlorobenzene at 110℃; for 6h; Reagent/catalyst; | 92% |

| Conditions | Yield |
|---|---|
| With phenylhydrazine In chloroform for 3h; Ambient temperature; | A 90% B 81% |

| Conditions | Yield |
|---|---|
| In ethanol at 75℃; for 7h; | 85% |
| In ethanol at 50 - 70℃; for 5h; Reflux; | 85% |
| With ammonia at 95℃; for 6h; pH=6; | 85% |
-
-
59-88-1
phenylhydrazine hydrochloride

-
-
141-97-9
ethyl acetoacetate

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
A
-
89-25-8
edaravone

-
B
-
61466-03-3, 4174-09-8
4,4-methine-bis-(3-methyl-1-phenyl-2-pyrazolin-5-one)

| Conditions | Yield |
|---|---|
| With p-toluenesulfonic acid monohydrate at 110℃; for 1h; Kinetics; Reagent/catalyst; Temperature; | A 78% B 12% |
| With pyridine hydrochloride at 140℃; for 24h; Kinetics; Reagent/catalyst; | A 39% B 47% |


-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 140℃; for 2h; Inert atmosphere; | 76% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; triethylamine; triphenylphosphine In tetrahydrofuran at 110℃; under 20521.4 Torr; for 10h; Temperature; Reagent/catalyst; Solvent; Time; Autoclave; regioselective reaction; | 72% |


| Conditions | Yield |
|---|---|
| With phenylhydrazine In ethanol | 67% |
| With phenylhydrazine In 1,4-dioxane; water | 11.17 g (64%) |

| Conditions | Yield |
|---|---|
| In ethanol at 50 - 80℃; for 7h; Temperature; Darkness; | 60.98% |
-
-
881-05-0
3-Methyl-1-phenyl-2-pyrazoline-4,5-dione

-
-
121-69-7
N,N-dimethyl-aniline

-
-
109-77-3
malononitrile

-
A
-
27781-29-9
bis-p-(N,N-dimethylaminophenyl)malonitrile

-
B
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| In ethanol for 2h; Heating; | A 60% B n/a |

-
-
387353-94-8
5-(fluoro-dimethylsilanyl)-3-methyl-1-phenyl-1H-pyrazole

-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With potassium fluoride; 3-chloro-benzenecarboperoxoic acid In N,N-dimethyl-formamide at -100℃; | 58% |
-
-
881-05-0
3-Methyl-1-phenyl-2-pyrazoline-4,5-dione

-
-
91-66-7
N,N-diethylaniline

-
-
109-77-3
malononitrile

-
A
-
27781-21-1
bis-p-(N,N-diethylaminophenyl)malonitrile

-
B
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| In ethanol for 2h; Heating; | A 55% B 42% |

-
-
130691-00-8
(4E)-4-benzylidene-5-methyl-2-phenyl-3,4-dihydropyrazol-3(3H)-one

-
-
137-07-5
2-amino-benzenethiol

-
A
-
883-93-2
2-Phenylbenzothiazole

-
B
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 3h; Heating; | A 25% B n/a C 55% |

-
-
50-00-0
formaldehyd

-
-
98395-57-4
1-Methoxymethyl-5-methyl-2-phenyl-1,2-dihydro-pyrazol-3-one

-
A
-
98395-58-5
C21H20N4O2

-
B
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 0.166667h; Heating; | A 53% B 46% |

-
-
98395-57-4
1-Methoxymethyl-5-methyl-2-phenyl-1,2-dihydro-pyrazol-3-one

-
A
-
98395-58-5
C21H20N4O2

-
B
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 0.166667h; Heating; | A 53% B 46% |
-
-
6287-35-0, 17469-23-7, 66558-23-4
3-phenylaminobut-2-enoic acid ethyl ester

-
-
59-88-1
phenylhydrazine hydrochloride

-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With potassium acetate In ethanol at 25℃; for 1h; | 50% |
-
-
897030-44-3
(E)-4-(4-chlorobenzylidene)-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one

-
-
137-07-5
2-amino-benzenethiol

-
A
-
6265-91-4
2-(4-chlorophenyl)benzothiazole

-
B
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 3h; Heating; | A 20% B n/a C 50% |

- polymer, product of polymerization of 4-vinylbenzyl-polytetrahydrofuran with styrene and 1,4-bis(4-vinylphenoxy)butane, with polytetrahydrofuran chain terminated with 2-phenylhydrazonopropylcarbonyloxy group
-
polymer, product of polymerization of 4-vinylbenzyl-polytetrahydrofuran with styrene and 1,4-bis(4-vinylphenoxy)butane, with polytetrahydrofuran chain terminated with 2-phenylhydrazonopropylcarbonyloxy group

-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In acetonitrile at 20℃; for 0.5h; | 48% |
- polymer, product derived from polytetrahydrofuran-grafted 5-hydroxypentyl-JandaJel (polytetrahydrofuran wt percent 33.4) with substituted polytetrahydrofuran chain end-OH group on 2-phenylhydrazonopropylcarbonyloxy group
-
polymer, product derived from polytetrahydrofuran-grafted 5-hydroxypentyl-JandaJel (polytetrahydrofuran wt percent 33.4) with substituted polytetrahydrofuran chain end-OH group on 2-phenylhydrazonopropylcarbonyloxy group

-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In acetonitrile at 20℃; for 0.5h; | 47% |

| Conditions | Yield |
|---|---|
| With piperidine In ethanol for 8h; Heating; | A n/a B 45% |


-
A
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With piperidine; cyclohexanone In ethanol for 8h; Heating; | A n/a B 45% |
-
-
132603-48-6
E-1-phenyl-3-methyl-4-<(4-nitrophenyl)methylene>-5-pyrazolone

-
-
137-07-5
2-amino-benzenethiol

-
A
-
22868-34-4
2-(4-nitrophenyl)benzothiazole

-
B
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol for 3h; Heating; | A 15% B n/a C 45% |

- polymer, product derived from polytetrahydrofuran-grafted 5-hydroxypentyl-JandaJel (polytetrahydrofuran wt percent 49.9) with substituted polytetrahydrofuran chain end-OH group on 2-phenylhydrazonopropylcarbonyloxy group
-
polymer, product derived from polytetrahydrofuran-grafted 5-hydroxypentyl-JandaJel (polytetrahydrofuran wt percent 49.9) with substituted polytetrahydrofuran chain end-OH group on 2-phenylhydrazonopropylcarbonyloxy group

-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In acetonitrile at 20℃; for 0.5h; | 42% |

| Conditions | Yield |
|---|---|
| With potassium acetate In ethanol at 25℃; for 0.75h; | 40% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 22h; | 40% |
-
-
70526-06-6
(Z)-3-pyrrolidin-1-yl-but-2-enoic acid ethyl ester

-
-
59-88-1
phenylhydrazine hydrochloride

-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With potassium acetate In ethanol at 25℃; for 1.41667h; | 21% |
-
-
22123-17-7
4-isopropylidene-5-methyl-2-phenyl-2,4-dihydro-pyrazol-3-one

-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With piperidine In benzene for 48h; Product distribution; Ambient temperature; | 8% |
-
-
121572-86-9
C22H18NO(1+)*I3(1-)

-
-
89-25-8
edaravone

-
-
121030-55-5
6,8-Dimethyl-7-[3-methyl-5-oxo-1-phenyl-1,5-dihydro-pyrazol-(4Z)-ylidene]-2,3-diphenyl-7H-indolizin-1-one

| Conditions | Yield |
|---|---|
| With pyridine for 1h; Ambient temperature; | 100% |

-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| Stage #1: 2-(4,6-dimethyl-3-cyano-2-pyridinylthio)benzenediazonium nitrate; 3-methyl-1-phenylpyrazolin-5-(4H)-one for 0.0833333h; grinding; Stage #2: With trimethylamine at 20℃; under 375.038 Torr; for 12h; Further stages.; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: α-bromoacetophenone; 3-methyl-1-phenylpyrazolin-5-(4H)-one at 20℃; for 1h; ball-milling; Stage #2: With sodium carbonate Further stages.; | 100% |
-
-
89-25-8
edaravone

-
-
22717-42-6
3-methyl-1-phenyl-1H-pyrazole-5(4H)-thione

| Conditions | Yield |
|---|---|
| With Lawessons reagent In toluene Reflux; | 100% |
| With Lawessons reagent In toluene for 2h; Reflux; | 97% |
| With Lawessons reagent for 0.0833333h; Irradiation; microwave; | 70% |
| With Lawessons reagent In toluene at 110℃; for 2h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: 4-methoxycarbonyl aniline With hydrogenchloride; sodium nitrite In water at 0 - 40℃; for 1h; Stage #2: 3-methyl-1-phenylpyrazolin-5-(4H)-one With sodium hydroxide In water at 2 - 5℃; for 1h; | 99.6% |

| Conditions | Yield |
|---|---|
| Stage #1: edaravone With potassium hydroxide In water; acetonitrile for 0.5h; Stage #2: benzoyl chloride In dichloromethane; water; acetonitrile for 0.0833333h; | 99% |
| With triethylamine In chloroform for 1h; Heating; | 90% |
| for 0.133333h; microwave irradiation; | 87% |

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform for 1h; Heating; | 99% |
| at 20℃; for 12h; | 65% |
| With 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one at 70 - 80℃; |
-
-
104-88-1
4-chlorobenzaldehyde

-
-
89-25-8
edaravone

-
-
109-77-3
malononitrile

-
-
76973-35-8
6-amino-4-(4-chlorophenyl)-5-cyano-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride In water at 90℃; for 6h; | 99% |
| With imidazole spacer and sulfonic acid tagged silica coated magnetite nanoparticle In neat (no solvent) at 20℃; for 0.333333h; Catalytic behavior; Reagent/catalyst; Temperature; | 98% |
| With Fe3O4 (at) tris(hydroxymethyl)aminomethane-SO3H nanoparticles In ethanol; water at 100℃; for 0.0833333h; | 98% |

-
-
100-52-7
benzaldehyde

-
-
89-25-8
edaravone

-
-
109-77-3
malononitrile

-
-
53316-57-7
6-amino-1,4-dihydro-3-methyl-1,4-diphenylpyrano[2,3-c]pyrazole-5-carbonitrile

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride In water at 90℃; for 6h; | 99% |
| With rac-Pro-OH at 25℃; for 0.0833333h; | 99% |
| With trichloroacetic acid at 100℃; for 0.05h; neat (no solvent); | 98% |

-
-
100-52-7
benzaldehyde

-
-
89-25-8
edaravone

-
-
57303-46-5
4,4'-(phenylmethylene)bis(3-methyl-1-phenyl-pyrazol-5-ol)

| Conditions | Yield |
|---|---|
| With MCM-41 nanoparticles-supported guanine bonded with zirconium(IV) In ethanol at 80℃; for 0.333333h; Green chemistry; chemoselective reaction; | 99% |
| With Mn/lysine(at)CoFe2O4 nanocomposite at 80℃; for 1h; | 99% |
| With 3-aminopropylated silica gel In acetonitrile at 20℃; for 0.166667h; tandem Knoevenagel-Michael reaction; | 98% |
-
-
123-11-5
4-methoxy-benzaldehyde

-
-
89-25-8
edaravone

-
-
109-77-3
malononitrile

-
-
53316-60-2
6-amino-4-(4-methoxyphenyl)-3-methyl-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile

| Conditions | Yield |
|---|---|
| With rac-Pro-OH at 25℃; for 0.166667h; | 99% |
| With sodium fluoride In methanol; water at 25℃; for 0.1h; Sonication; | 98% |
| With N-benzyl-N,N,N-triethylammonium chloride In water at 90℃; for 6h; | 96% |

-
-
110719-45-4
1-(3-methyl-2-butenyl)-pyrrol-2-carbaldehyde

-
-
89-25-8
edaravone

-
-
110719-46-5
2,4-dihydro-5-methyl-4-<1'-(3-methyl-2-butenyl)pyrrol-2-yl>methylene-2-phenyl-3H-pyrazol-3-one

| Conditions | Yield |
|---|---|
| With ethylenediamine diacetic acid In acetonitrile | 99% |

| Conditions | Yield |
|---|---|
| With trimethylamine at 20℃; under 375.038 Torr; for 12h; | 99% |

-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With trimethylamine at 20℃; under 375.038 Torr; for 12h; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: edaravone With potassium hydroxide In water; acetonitrile for 0.5h; Stage #2: 4-methyl-benzoyl chloride In dichloromethane; water; acetonitrile for 0.0833333h; | 99% |
| for 0.133333h; microwave irradiation; | 37% |

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform for 1h; Heating; | 99% |
| for 0.133333h; microwave irradiation; | 81% |
| With 1,1-dichloroethane; triethylamine at 50 - 60℃; |
-
-
104-87-0
4-methyl-benzaldehyde

-
-
89-25-8
edaravone

-
-
109-77-3
malononitrile

-
-
53316-59-9
3-methyl-6-amino-5-cyano-4-(4-methylphenyl)-1-phenyl-1,4-dihydropyrano[2,3-c]pyrazole

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride In water at 90℃; for 6h; | 99% |
| With imidazole spacer and sulfonic acid tagged silica coated magnetite nanoparticle In neat (no solvent) at 20℃; for 0.75h; | 94% |
| With [amberlite IRA900OH]-supported L-prolinate In ethanol for 0.3h; Catalytic behavior; Reflux; | 94% |


| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride In water at 90℃; for 6h; | 99% |
| With imidazole spacer and sulfonic acid tagged silica coated magnetite nanoparticle In neat (no solvent) at 20℃; for 0.25h; | 97% |
| With triethylammonium acetate at 20℃; for 0.5h; | 96% |

-
-
65-22-5
pyridoxal hydrochloride

-
-
89-25-8
edaravone

-
-
854010-13-2
1-(5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)-6-methyl-1,3-dihydro-furo[3,4-c]pyridin-7-ol

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 10 - 20℃; pH=2.04; Reagent/catalyst; pH-value; | 99% |
| With sodium hydroxide In water at 20℃; for 0.5h; | 6.8% |
| With sodium hydroxide In water at 20 - 30℃; for 2h; pH=6.3 - 12.1; Time; |

| Conditions | Yield |
|---|---|
| In ethanol at 78℃; for 0.0833333h; Green chemistry; | 99% |
| With 2-carbamoylhydrazine-1-sulfonic acid In neat (no solvent) at 60℃; for 0.416667h; Reagent/catalyst; Green chemistry; | 97% |
| With phosphomolybdic acid In ethanol at 20℃; for 4h; | 94% |
-
-
37595-74-7
N,N-phenylbistrifluoromethane-sulfonimide

-
-
89-25-8
edaravone

-
-
276249-49-1
trifluoromethanesulfonic acid 5-methyl-2-phenyl-2H-pyrazol-3-yl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 35℃; for 3h; | 99% |
-
-
102-96-5
(2-nitroethenyl)benzene

-
-
89-25-8
edaravone

-
-
1440200-00-9
(R)-5-methyl-4-(2-nitro-1-phenylethyl)-2-phenyl-2H-pyrazol-3-ol

| Conditions | Yield |
|---|---|
| With quinine In chloroform at 20℃; for 4h; Solvent; Temperature; Reagent/catalyst; Michael Addition; enantioselective reaction; | 99% |
| With C33H30F6N4O3 In dichloromethane at 20℃; for 24h; Michael Addition; Inert atmosphere; enantioselective reaction; | 96% |
| With C31H39N3O9S; benzoic acid In toluene at 20℃; Inert atmosphere; | 95% |
| With C29H32N4O2S In chloroform at -30℃; for 12h; Michael Addition; enantioselective reaction; | 95.3% |
| With C40H31F6N3O2 In 1,2-dichloro-ethane at 20℃; for 24h; Catalytic behavior; Reagent/catalyst; Solvent; Michael Addition; enantioselective reaction; | 93% |
-
-
706-07-0
1-chloro-4-(2-nitrovinyl)benzene

-
-
89-25-8
edaravone

-
-
1451195-14-4
(R)-4-(1-(4-chlorophenyl)-2-nitroethyl)-3-methyl-1-phenyl-1H-pyrazol-5-ol

| Conditions | Yield |
|---|---|
| With quinine In chloroform at 20℃; for 4h; Michael Addition; enantioselective reaction; | 99% |
| With C31H39N3O9S; benzoic acid In toluene at 20℃; for 12h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 99% |
| With C40H31F6N3O2 In 1,2-dichloro-ethane at 20℃; for 18h; Michael Addition; enantioselective reaction; | 95% |
| With C29H32N4O2S In chloroform at -30℃; for 12h; Michael Addition; enantioselective reaction; | 93.7% |
| With C31H39N3O9S; benzoic acid In toluene at 20℃; Inert atmosphere; |
-
-
623-27-8
terephthalaldehyde,

-
-
89-25-8
edaravone

-
-
306765-12-8
4,4'-(1,4-phenylenebis(methanylylidene))bis(3-methyl-1-phenyl-1H-pyrazol)-5(4H)-one

| Conditions | Yield |
|---|---|
| With sulfuric acid-modified polyethyleneglycol-6000 In neat (no solvent) at 70℃; for 0.05h; Knoevenagel Condensation; Green chemistry; | 99% |
-
-
882-26-8
3-nitro-β-nitrostyrene

-
-
89-25-8
edaravone

-
-
1451195-21-3
(R)-3-methyl-4-(2-nitro-1-(3-nitrophenyl)ethyl)-1-phenyl-1H-pyrazol-5-ol

| Conditions | Yield |
|---|---|
| With quinine In chloroform at 20℃; for 6h; Michael Addition; enantioselective reaction; | 99% |
| With C29H32N4O2S In chloroform at -30℃; for 18h; Michael Addition; enantioselective reaction; | 93.2% |
| With C40H31F6N3O2 In 1,2-dichloro-ethane at 20℃; for 24h; Michael Addition; enantioselective reaction; | 92% |
-
-
50598-92-0
2-cyclohexyl-1-nitroethene

-
-
89-25-8
edaravone

-
-
1451195-27-9
(R)-4-(1-cyclohexyl-2-nitro-ethyl)-5-methyl-2-phenyl-2H-pyrazol-3-ol

| Conditions | Yield |
|---|---|
| With quinine In chloroform at 20℃; for 12h; Michael Addition; enantioselective reaction; | 99% |
-
-
5153-67-3
nitrostyrene

-
-
89-25-8
edaravone

-
-
1440200-00-9
(R)-5-methyl-4-(2-nitro-1-phenylethyl)-2-phenyl-2H-pyrazol-3-ol

| Conditions | Yield |
|---|---|
| With C31H39N3O9S; benzoic acid In toluene at 20℃; for 12h; Reagent/catalyst; Solvent; Time; Schlenk technique; Inert atmosphere; enantioselective reaction; | 99% |
| Stage #1: nitrostyrene With (S)-3-(3,5-bis(trifluoromethyl)phenylamino)-4-(1-phenyl-2-(piperidin-1-yl)ethylamino)cyclobut-3-ene-1,2-dione In 1,2-dichloro-ethane for 0.166667h; Michael Addition; Inert atmosphere; Stage #2: edaravone In 1,2-dichloro-ethane at 20℃; for 24h; Michael Addition; Inert atmosphere; enantioselective reaction; | n/a |
-
-
66-77-3
1-naphthaldehyde

-
-
89-25-8
edaravone

-
-
1433750-55-0
4,4'-(naphthalen-1-ylmethanediyl)bis(3-methyl-1-phenyl-1H-pyrazol-5-ol)

| Conditions | Yield |
|---|---|
| With 2-carbamoylhydrazine-1-sulfonic acid In neat (no solvent) at 60℃; for 0.75h; Reagent/catalyst; Green chemistry; | 99% |
| With zinc(II) oxide In ethanol; water for 0.5h; Catalytic behavior; Reflux; | 93% |
| Stage #1: 1-naphthaldehyde; 3-methyl-1-phenylpyrazolin-5-(4H)-one With zinc(II) oxide In ethanol; water Knoevenagel Condensation; Reflux; Green chemistry; Stage #2: With zinc(II) oxide In ethanol; water Michael Addition; Reflux; Green chemistry; | 90% |

| Conditions | Yield |
|---|---|
| With quinine In dichloromethane at -15℃; for 96h; Catalytic behavior; Solvent; Temperature; Reagent/catalyst; Michael Addition; enantioselective reaction; | 99% |
Edaravone Chemical Properties
Product Name: Norphenazone (CAS NO.89-25-8)
IUPAC Name: 5-methyl-2-phenyl-4H-pyrazol-3-one
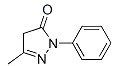
Molecular formula: C10H10N2O
Molar mass: 174.2
EINECS: 201-891-0
Melting point: 126-128°C
Boiling point: 287°C 265 mm Hg
Density: 1.12 g/cm3
Flash point: 191°C 17mm
Water Solubility: 3 g/L (20°C)
Stability: stable under ordinary conditions. Light sensitive
Product Categories: Intermediates of Dyes and Pigments; API; Aromatics Compounds; Aromatices; Heterocycles; Intermediates & Fine Chemicals; Pharmaceuticals; ChromophoresPeptide Synthesis; Glycan Labeling; Glycan Labeling and Analysis; OthersDerivatization Reagents; Coupling;Derivatization Reagents HPLC; OthersSynthetic Reagents; Peptide Synthesis; UV-VIS
Edaravone Uses
Norphenazone (CAS NO.89-25-8) possibly can be used in the synthesis of dyes, drugs, pesticides and other organic compounds.
Edaravone Toxicity Data With Reference
| 1. | eye-rbt 500 mg/24H MOD | 28ZPAK Sbornik Vysledku Toxixologickeho Vysetreni Latek A Pripravku Marhold, J.V.,Institut Pro Vychovu Vedoucicn Pracovniku Chemickeho Prumyclu Praha,Czechoslovakia.: 1972,144. | ||
| 2. | orl-rat LD50:3500 mg/kg | LONZA# Personal Communication from LONZA Ltd., CH-4002, Basel, Switzerland, to NIOSH, Cincinnati, OH 45226 08FEB79 . | ||
| 3. | ipr-mus LD50:2012 mg/kg | RPTOAN Russian Pharmacology and Toxicology. Translation of FATOAO. 36 (1973),27. |
Edaravone Consensus Reports
NCI Carcinogenesis Bioassay (feed); No Evidence: mouse, rat NCITR* National Cancer Institute Carcinogenesis Technical Report Series. (Bethesda, MD 20014) No. NCI-CG-TR-141 ,1978. . Reported in EPA TSCA Inventory.
Edaravone Safety Profile
Moderately toxic by ingestion and intraperitoneal routes. An eye irritant. When heated to decomposition it emits toxic fumes of NOx.
Safety Information of Norphenazone (CAS NO.89-25-8):
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 26-36
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
WGK Germany: 1
Edaravone Specification
Norphenazone, its CAS NO. is 89-25-8, the synonyms are 2,4-dihydro-5-methyl-2-phenyl-3h-pyrazol-3-one ; 5-Methyl-2-phenyl-1,2-dihydropyrazol-3-one .
Related Products
- Edaravone
- 892596-78-0
- 89260-45-7
- 89260-46-8
- 89265-09-8
- 89265-35-0
- 892664-04-9
- 89267-81-2
- 89-26-9
- 89269-64-7
- 89277-99-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View