-
Name
Ethyl difluoroacetate
- EINECS 207-223-4
- CAS No. 454-31-9
- Article Data46
- CAS DataBase
- Density 1.135 g/cm3
- Solubility Slightly soluble in water
- Melting Point
- Formula C4H6F2O2
- Boiling Point 76 °C at 760 mmHg
- Molecular Weight 124.087
- Flash Point 1.1 °C
- Transport Information UN 1992
- Appearance clear colorless liquid
- Safety 45-36/37/39-26-16-27
- Risk Codes 34-10
-
Molecular Structure
-
Hazard Symbols
 C,
C,  F,
F,  T,
T,  Xi
Xi
- Synonyms Aceticacid, difluoro-, ethyl ester (6CI,7CI,8CI,9CI);2,2-Difluoroacetic acid ethylester;Difluoroacetic acid ethyl ester;Ethyl 2,2-difluoroacetate;Ethyl difluoroacetic;
- PSA 26.30000
- LogP 0.81460
Synthetic route

| Conditions | Yield |
|---|---|
| With 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-7-methylbenzo-[d]imidazole; [Zr(2,6-bis(5-methyl-3-phenyl-1H-pyrrol-2-yl)pyridine)2] In benzene-d6 for 2.5h; Catalytic behavior; UV-irradiation; Inert atmosphere; Schlenk technique; | 98% |
| With 1,2,3-trimethoxybenzene; (3,5-dimethyl-2-(2-pyridyl)pyrrolide)3ZrCl; 1,3-dimethyl-2-phenyl-2,3-dihydro-1H-7-methylbenzo-[d]imidazole In benzene-d6 at 33℃; for 0.5h; Time; Irradiation; Glovebox; | 85% |
| With N,N,N,N,-tetramethylethylenediamine In dimethylsulfoxide-d6 at 20℃; for 15h; Irradiation; | 41% |
| Stage #1: Ethyl bromodifluoroacetate With zinc In tetrahydrofuran at 20 - 50℃; for 1h; Inert atmosphere; Stage #2: With hydrogenchloride In tetrahydrofuran; water at 0℃; Product distribution / selectivity; | ~ 90 %Chromat. |
| With hydrogen; nickel; triethylamine In ethanol at 80℃; under 3750.38 - 7500.75 Torr; |

| Conditions | Yield |
|---|---|
| With N,N-dimethyl acetamide for 4h; Product distribution / selectivity; Inert atmosphere; Sealed tube; Cooling with ice; | 96% |
| With sodium carbonate at 5 - 20℃; for 1h; Product distribution / selectivity; Inert atmosphere; | 77% |
| at 20℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 0 - 70℃; for 2h; Temperature; | 93.98% |
-
-
36589-84-1
1,1-diethoxy-2,2-difluoro-ethane

-
-
454-31-9
ethyl difluoroacetate

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; vanadia at 5℃; for 5h; | 89.9% |

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 54 - 56℃; for 1h; | 87% |
| Stage #1: Ethyl 1,1,2,2-tetrafluoroethyl ether With sulfuric acid; hydrogen fluoride In ethanol; water at -20 - 50℃; for 24h; Autoclave; Stage #2: With sodium hydrogencarbonate at 20℃; under 760.051 Torr; for 1h; Reagent/catalyst; | 86% |
| With nitric acid | |
| With sulfuric acid | |
| With water; fluorinated γ-alumina at 180℃; for 0.0202778h; Product distribution / selectivity; Inert atmosphere; Gas phase; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; silica gel at 50℃; for 24h; Time; Autoclave; | 86% |

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; triethylamine In ethanol at 30℃; under 750.075 - 3000.3 Torr; | 75% |
| With zinc In N,N-dimethyl-formamide at 70℃; Yield given; |
-
-
592-76-7
1-Heptene

-
-
7648-30-8
ethyl 2,2-difluoro-2-iodoacetate

-
A
-
454-31-9
ethyl difluoroacetate

-
B
-
127224-10-6
Ethyl 2,2-Difluoro-4-iodononanoate

| Conditions | Yield |
|---|---|
| With hydroquinone; nickel dichloride; zinc In tetrahydrofuran; water 1) RT, 15 min, 2) RT, 20 min; | A 70% B 17% |

-
-
383-62-0
ethyl 2-chloro-2,2-difluoroacetate

-
A
-
381-73-7
Difluoroacetic acid

-
B
-
454-31-9
ethyl difluoroacetate

-
C
-
76-04-0
2-chloro-2,2-difluoroacetic acid

| Conditions | Yield |
|---|---|
| With ethanol; zinc for 1 - 3h; Product distribution / selectivity; Heating / reflux; | A 9.7% B 61.6% C 0.4% |


| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 10 - 120℃; for 14h; | 57% |
-
-
617-35-6
2-oxo-propionic acid ethyl ester

-
-
667-27-6
Ethyl bromodifluoroacetate

-
A
-
454-31-9
ethyl difluoroacetate

| Conditions | Yield |
|---|---|
| Stage #1: 2-oxo-propionic acid ethyl ester; Ethyl bromodifluoroacetate In N,N-dimethyl-formamide at -20℃; Stage #2: With Tetrakis(dimethylamino)ethylen In N,N-dimethyl-formamide at -20 - 20℃; | A n/a B 25% |

-
-
667-27-6
Ethyl bromodifluoroacetate

-
-
58837-16-4
1,3,5-tris(4-methoxybenzyl)hexahydro-1,3,5-triazine

-
A
-
454-31-9
ethyl difluoroacetate

| Conditions | Yield |
|---|---|
| Stage #1: Ethyl bromodifluoroacetate With chloro-trimethyl-silane; zinc In tetrahydrofuran for 0.416667h; Stage #2: 1,3,5-tris(4-methoxybenzyl)hexahydro-1,3,5-triazine In tetrahydrofuran at 25℃; for 3h; | A n/a B 25% C n/a D n/a |


| Conditions | Yield |
|---|---|
| With potassium fluoride In ethanol; water at 100℃; for 40h; Solvent; Concentration; | 21% |
| With potassium fluoride; C6H9BO4 In ISOPROPYLAMIDE at 20 - 75℃; Halex reaction; |
-
-
75-77-4
chloro-trimethyl-silane

-
-
383-63-1
ethyl trifluoroacetate,

-
A
-
454-31-9
ethyl difluoroacetate

-
B
-
205865-67-4
α-(trimethylsilyl)difluoroacetic acid ethyl ester

-
C
-
565214-84-8
trifluoroacetyltrimethylsilane ethyltrimethylsilyl ketal

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide In tetrahydrofuran at -25℃; Electrochemical reaction; Further byproducts given; | A 8% B 8 % Spectr. C 33 % Spectr. D 1 % Spectr. |


| Conditions | Yield |
|---|---|
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With fluorine at 100℃; ueber Kupferdrahtnetz und Umsetzen der Reaktionsprodukte mit absol.Aethanol; |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
60-29-7
diethyl ether

-
-
17976-35-1
1,1,2,3,4,4-hexafluoro-2-butene

-
A
-
433-53-4
difluoroacetic acid methyl ester

-
B
-
454-31-9
ethyl difluoroacetate

| Conditions | Yield |
|---|---|
| With potassium hydroxide; potassium permanganate 1.) 60 degC, water; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With N-(2-chloro-1,1,2-trifluoroethyl)diethylamine; sodium fluoride 1.) -78 deg C, 15 min, 2.) triglyme; Yield given. Multistep reaction; |
-
-
383-63-1
ethyl trifluoroacetate,

-
A
-
74-84-0
ethane

-
B
-
144-49-0
Fluoroacetic acid

-
C
-
459-72-3
ethyl 2-fluoroacetate

-
D
-
454-31-9
ethyl difluoroacetate

-
E
-
141-78-6
ethyl acetate

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide for 2h; Quantum yield; Irradiation; |

-
-
557-99-3
acetyl fluoride

-
A
-
459-72-3
ethyl 2-fluoroacetate

-
B
-
454-31-9
ethyl difluoroacetate

-
C
-
383-63-1
ethyl trifluoroacetate,

| Conditions | Yield |
|---|---|
| at 100℃; Umsetzen der Reaktionsprodukte mit absol.Aethanol bei -80grad,zuletzt bei Siedetemperatur; |

-
-
780-25-6
N-benzylidene benzylamine

-
-
205865-67-4
α-(trimethylsilyl)difluoroacetic acid ethyl ester

-
A
-
454-31-9
ethyl difluoroacetate

-
B
-
53986-95-1
N-benzylidene-α-(trimethylsilyl)benzylamine

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; potassium fluoride at 50℃; for 12h; |

-
-
667-27-6
Ethyl bromodifluoroacetate

-
-
140-88-5
ethyl acrylate

-
A
-
454-31-9
ethyl difluoroacetate

-
B
-
428-97-7
2,2-difluoroglutaric acid diethyl ester

| Conditions | Yield |
|---|---|
| With N,N,N,N,-tetramethylethylenediamine; water; copper In tetrahydrofuran Michael Addition; | A 10 %Spectr. B 52 %Spectr. |

-
-
667-27-6
Ethyl bromodifluoroacetate

-
-
98-80-6
phenylboronic acid

-
A
-
454-31-9
ethyl difluoroacetate

-
B
-
2248-46-6
ethyl 2,2-difluoro-2-phenylacetate

| Conditions | Yield |
|---|---|
| With 2,2':6,2''-terpyridine; nickel chloride dimethyl ether; potassium carbonate In 1,4-dioxane at 80℃; for 8h; Schlenk technique; Inert atmosphere; | A 7 %Spectr. B 1 %Spectr. |
| With [2,2]bipyridinyl; [1,1'-bis(diphenylphosphino)ferrocene]nickel(II) chloride; potassium carbonate In 1,4-dioxane at 80℃; for 8h; Reagent/catalyst; Solvent; Schlenk technique; Inert atmosphere; | A 6 %Spectr. B 89 %Spectr. |

-
-
88-12-0
1-ethenyl-2-pyrrolidinone

-
-
667-27-6
Ethyl bromodifluoroacetate

-
-
5122-94-1
4-biphenylboronic acid

-
A
-
454-31-9
ethyl difluoroacetate

-
C
-
73789-98-7
2-([1,1'-biphenyl]-4-yl)-2,2-difluoroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With (1,2-dimethoxyethane)dichloronickel(II); 4,4'-dibromo-2,2'-bipyridine; potassium carbonate In tetrahydrofuran at 80℃; for 24h; Reagent/catalyst; Solvent; Schlenk technique; Inert atmosphere; | A 15 %Spectr. B 53 %Spectr. C 13 %Spectr. |

-
-
88-12-0
1-ethenyl-2-pyrrolidinone

-
-
667-27-6
Ethyl bromodifluoroacetate

-
-
5122-94-1
4-biphenylboronic acid

-
A
-
454-31-9
ethyl difluoroacetate

-
D
-
73789-98-7
2-([1,1'-biphenyl]-4-yl)-2,2-difluoroacetic acid ethyl ester

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)nickel(II) chloride; 4,4'-Dimethoxy-2,2'-bipyridin; potassium carbonate In tetrahydrofuran at 80℃; for 24h; Concentration; Reagent/catalyst; Solvent; Schlenk technique; Inert atmosphere; | A 13 %Spectr. B 49 %Spectr. C 7 %Spectr. D 36 %Spectr. |

-
-
383-62-0
ethyl 2-chloro-2,2-difluoroacetate

-
A
-
359-13-7
difluoroethanol

-
B
-
454-31-9
ethyl difluoroacetate

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; triethylamine In ethanol at 45℃; under 7500.75 - 30003 Torr; for 5h; Temperature; Time; Pressure; |

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen; triethylamine In tetrahydrofuran at 100℃; under 750.075 - 15001.5 Torr; | |
| With diethylzinc In hexane; [D3]acetonitrile at -20℃; for 8h; Schlenk technique; Inert atmosphere; |
-
-
454-31-9
ethyl difluoroacetate

-
-
148992-43-2
1-ethoxy-2,2-difluoroethanol

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran; diethyl ether at -78℃; for 2.75h; | 100% |
| With lithium aluminium tetrahydride In tetrahydrofuran; diethyl ether at -78℃; for 3h; | 60% |
| With lithium aluminium tetrahydride In tetrahydrofuran; diethyl ether at -78℃; for 3h; Reagent/catalyst; Solvent; | 60% |
-
-
454-31-9
ethyl difluoroacetate


| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 16h; | 100% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In toluene at 0 - 20℃; for 2h; Inert atmosphere; | 100% |
| With potassium tert-butylate In toluene at 0 - 20℃; for 2h; Inert atmosphere; | 100% |
| With potassium tert-butylate In toluene at 0 - 20℃; Inert atmosphere; | 100% |
| With potassium tert-butylate In toluene at 0 - 20℃; for 2h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In toluene at 0 - 20℃; for 2h; Inert atmosphere; | 100% |
-
-
681425-32-1
(S)-{(1α,5α,6α)-3-[4-(5-aminomethyl-2-oxooxazolidin-3-yl)-2,6-difluorophenyl]-3-azabicyclo[3.1.0]hex-6-yl}carbamic acid tert-butyl ester

-
-
454-31-9
ethyl difluoroacetate

-
-
681425-33-2
(S)-[(1α,5α,6α)-3-(4-{5-[(2,2-difluoroacetylamino)methyl]-2-oxooxazolidin-3-yl}-2,6-difluorophenyl)-3-azabicyclo[3.1.0]hex-6-yl]carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; for 15h; | 99% |
| With triethylamine In methanol at 20℃; for 15h; | 99% |
-
-
454-31-9
ethyl difluoroacetate

-
-
97004-04-1
5-(aminomethyl)-2-chloropyridine

-
-
1135440-09-3
N-(2-chloro-5-aminomethylpyridine)-1,1-difluoroacetamide

| Conditions | Yield |
|---|---|
| at 100℃; for 1h; Reflux; | 99% |
-
-
454-31-9
ethyl difluoroacetate

-
-
76-05-1
trifluoroacetic acid

-
A
-
381-73-7
Difluoroacetic acid

-
B
-
383-63-1
ethyl trifluoroacetate,

| Conditions | Yield |
|---|---|
| at 85℃; Product distribution / selectivity; | A 99% B n/a |


| Conditions | Yield |
|---|---|
| With potassium tert-butylate In toluene at 0 - 20℃; for 2h; Inert atmosphere; | 99% |
| With potassium tert-butylate In toluene at 0 - 20℃; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In toluene at 0 - 20℃; Inert atmosphere; | 99% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
119-61-9
benzophenone

-
-
454-31-9
ethyl difluoroacetate

-
-
14629-59-5
diphenylmethyl trimethylsilyl ether

| Conditions | Yield |
|---|---|
| With magnesium In 1-methyl-pyrrolidin-2-one at 20℃; Inert atmosphere; | 98% |

-
-
858643-92-2
tert-butyl 3-acetylpiperidine-1-carboxylate

-
-
454-31-9
ethyl difluoroacetate

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In benzene at 0 - 20℃; for 5h; Inert atmosphere; | 98% |
| With potassium tert-butylate In benzene at 0 - 20℃; for 5h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| In dichloromethane for 16h; Reflux; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| In dichloromethane for 16h; Reflux; Inert atmosphere; | 98% |
-
-
454-31-9
ethyl difluoroacetate

-
-
484-51-5
5-acetyl-6-hydroxy-4,7-dimethoxybenzofuran

-
-
376384-40-6
7-(difluoromethyl)-7-hydroxy-4,9-dimethoxy-6,7-dihydro-5H-furo[3,2-g]chromen-5-one

| Conditions | Yield |
|---|---|
| With lithium hydride In tetrahydrofuran Heating; | 97% |
-
-
454-31-9
ethyl difluoroacetate

-
-
683-08-9
Diethyl methylphosphonate

-
-
1023327-32-3
diethyl (3,3-difluoro-2-oxopropyl)phosphonate

| Conditions | Yield |
|---|---|
| Stage #1: Diethyl methylphosphonate With n-butyllithium In tetrahydrofuran; hexane at -95℃; for 1h; Inert atmosphere; Stage #2: ethyl difluoroacetate In tetrahydrofuran; hexane at -95 - -90℃; for 1h; Inert atmosphere; | 97% |
| Stage #1: Diethyl methylphosphonate With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 1h; Stage #2: ethyl difluoroacetate In tetrahydrofuran at -78 - 0℃; Further stages.; | 82% |
| With n-butyllithium In tetrahydrofuran; hexane at -78 - -65℃; for 3h; |

| Conditions | Yield |
|---|---|
| With ammonia In ethanol at 0 - 25℃; for 2.5h; | 96% |
| With ammonia |
-
-
454-31-9
ethyl difluoroacetate

-
-
455-91-4
1-(3-fluoro-4-methoxyphenyl)ethanone

-
-
170570-77-1
4,4-difluoro-1-(3-fluoro-4-methoxyohenyl)-butane-1,3-dione

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium methylate In tert-butyl methyl ether | 96% |
| With hydrogenchloride; sodium methylate In tert-butyl methyl ether | 96% |
| With hydrogenchloride; sodium methylate In tert-butyl methyl ether | 96% |
-
-
64-18-6
formic acid

-
-
454-31-9
ethyl difluoroacetate

-
A
-
381-73-7
Difluoroacetic acid

-
B
-
109-94-4
formic acid ethyl ester

| Conditions | Yield |
|---|---|
| sulfuric acid at 70℃; Product distribution / selectivity; | A 96% B n/a |


| Conditions | Yield |
|---|---|
| Stage #1: ethyl acetate With sodium ethanolate at 25℃; Stage #2: ethyl difluoroacetate at 10 - 65℃; Stage #3: With sulfuric acid; water at 20 - 25℃; for 0.333333h; Product distribution / selectivity; | 95.5% |
| With multielement hydrotalcite at 55℃; for 8h; Catalytic behavior; Temperature; Reagent/catalyst; Green chemistry; | 88.9% |
| With lithium diisopropyl amide In diethyl ether at -70℃; for 4h; | 82% |
-
-
454-31-9
ethyl difluoroacetate

-
-
536-74-3
phenylacetylene

-
-
117710-72-2
1,1-difluoro-4-phenyl-3-butyn-2-one

| Conditions | Yield |
|---|---|
| Stage #1: phenylacetylene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 3h; Inert atmosphere; Stage #2: ethyl difluoroacetate With boron trifluoride diethyl etherate In tetrahydrofuran; hexane at -78℃; for 2h; Inert atmosphere; | 95% |
| With n-butyllithium; boron trifluoride diethyl etherate 1.) THF, -78 deg C, 30 min, 2.) -78 deg C, 90 min; Yield given. Multistep reaction; | |
| With n-butyllithium; boron trifluoride diethyl etherate 1.) hexane, THF, -78 deg C, 30 min, 2.) THF, hexane, -78 deg C, 1.5 h; Yield given. Multistep reaction; | |
| With n-butyllithium; boron trifluoride diethyl etherate |
-
-
51-17-2
benzoimidazole

-
-
454-31-9
ethyl difluoroacetate

-
-
84941-15-1
1-(difluoromethyl)-1H-benzo[d]imidazole

| Conditions | Yield |
|---|---|
| With potassium hydroxide In acetonitrile at 60℃; for 6h; Concentration; Solvent; Temperature; Sealed tube; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogen at 110℃; under 3750.38 - 15001.5 Torr; for 4h; Temperature; Pressure; Autoclave; | 93.3% |
| With C39H53ClN2P2Ru; potassium methanolate; hydrogen In tetrahydrofuran at 20℃; under 38002.6 Torr; for 16h; Glovebox; Autoclave; | 93% |
| With lithium aluminium tetrahydride In tetrahydrofuran at -10℃; for 1h; | 44.6% |
-
-
454-31-9
ethyl difluoroacetate

-
-
23699-74-3
1-(2-anilinophenyl)ethan-1-one

-
-
837364-34-8
2-(difluoromethyl)-1-phenylquinolin-4(1H)-one

| Conditions | Yield |
|---|---|
| With lithium hydride In tetrahydrofuran for 1h; Heating; | 93% |
-
-
454-31-9
ethyl difluoroacetate

-
-
54696-05-8
4'-benzyloxy-acetophenone

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol | 93% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium methylate | 93% |
| With hydrogenchloride; sodium methylate | 93% |

| Conditions | Yield |
|---|---|
| With dmap In methanol at 20℃; for 12h; | 92.8% |
Ethyl difluoroacetate Chemical Properties
IUPAC Name: Ethyl 2,2-difluoroacetate
Following is the structure of Aceticacid, 2,2-difluoro-, ethyl ester (CAS NO.454-31-9):
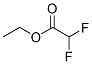
Molecular Formula: C4H6F2O2
Molecular Weight: 124.09g/mol
Mol File: 454-31-9.mol
EINECS: 207-223-4
Flash Point: 1.1 °C
Density: 1.135 g/cm3
Refractive index: 1.3465-1.3485
Surface Tension: 20.4 dyne/cm
Boiling point: 76 °C at 760 mmHg
Enthalpy of Vaporization: 31.71 kJ/mol
Vapour Pressure: 103 mmHg at 25°C
XLogP3-AA: 0.9
H-Bond Acceptor: 4
Molar Refractivity: 22.64 cm3
Molar Volume: 109.3 cm3
Appearance of Aceticacid, 2,2-difluoro-, ethyl ester (CAS NO.454-31-9): Colorless to light brown clear liquid
Product Categories of Aceticacid, 2,2-difluoro-, ethyl ester (CAS NO.454-31-9): Pharmaceutical Intermediates; Fluoro-Aliphatics ; Organic Fluorides; Fluorochemicals; Fluorinated Building Blocks; Fluorinating Reagents & Building Blocks for Fluorinated Biochemical Compounds; Synthetic Organic Chemistry; C2 to C5; Carbonyl Compounds; Esters
Canonical SMILES: CCOC(=O)C(F)F
InChI: InChI=1S/C4H6F2O2/c1-2-8-4(7)3(5)6/h3H,2H2,1H3
InChIKey: GZKHDVAKKLTJPO-UHFFFAOYSA-N
Ethyl difluoroacetate Safety Profile
Safety Information of Aceticacid, 2,2-difluoro-, ethyl ester (CAS NO.454-31-9):
Hazard Codes: C ,F
,F ,T
,T ,Xi
,Xi
Risk Statements: 34-10
10: Flammable
34: Causes burns
Safety Statements: 45-36/37/39-26-16-27
16: Keep away from sources of ignition - No smoking
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
27: Take off immediately all contaminated clothing
36: Wear suitable protective clothing
37: Wear suitable gloves
39: Wear eye/face protection
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
RIDADR: 1992
Hazard Note: Flammable
HazardClass: 3
PackingGroup: III
Ethyl difluoroacetate Specification
Aceticacid, 2,2-difluoro-, ethyl ester , its CAS NO. is 454-31-9, the synonyms are Ethyl difluoroacetate ; and Acetic acid, difluoro-, ethyl ester .
Related Products
- Ethyl (13-cis)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoate
- ethyl (1R,2R)-1-phenyl-2-(trideuteriomethylamino)cyclohex-3-ene-1-carboxylate,hydrochloride
- Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate hydrochloride
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (2-bromopropionamido)acetate
- Ethyl (2-cyanoimino-5,6-dichloro-1,2,3,4-tetrahydroquinazolin-3-yl)acetate
- ETHYL (2E,4Z)-DECADIENOATE
- Ethyl (2-hydroxyethyl)dimethyl-ammonium benzilate chloride
- Ethyl (2-mercaptoethyl) carbamate S-ester with O,O-dimethyl phosphorodithioate
- 45434-02-4
- 4543-47-9
- 4543-66-2
- 45438-73-1
- 45438-77-5
- 45438-80-0
- 4544-20-1
- 454451-28-6
- 454473-66-6
- 454476-59-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View