-
Name
Ethyl nicotinoate
- EINECS 210-370-7
- CAS No. 614-18-6
- Article Data117
- CAS DataBase
- Density 1.102 g/cm3
- Solubility Miscible with water
- Melting Point 8-10 °C(lit.)
- Formula C8H9NO2
- Boiling Point 224 °C at 760 mmHg
- Molecular Weight 151.165
- Flash Point 93.3 °C
- Transport Information
- Appearance clear colourless to yellow liquid
- Safety 26-39-37/39-36
- Risk Codes 37/38-41-36/38-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Nicotinicacid, ethyl ester (6CI,8CI);3-(Ethoxycarbonyl)pyridine;3-Carbethoxypyridine;Ethyl 3-pyridinecarboxylate;Ethyl nicotinate;Ignicut;Ignocut;Mucotherm;Nicaethan;Nikethan;Nikithan;b-Pyridinecarboxylic acid ethyl ester;
- PSA 39.19000
- LogP 1.25830
Synthetic route
-
-
1120-90-7
3-iodopyridine

-
-
64-17-5
ethanol

-
-
201230-82-2
carbon monoxide

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; palladium diacetate at 20 - 125℃; under 7500.75 - 22502.3 Torr; for 0.333333h; microwave irradiation; | 98% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene at 80℃; under 760.051 Torr; for 6h; | 94% |
| With palladium diacetate; 1,8-diazabicyclo[5.4.0]undec-7-ene at 120℃; under 9308.91 Torr; | |
| With palladium diacetate; 1,8-diazabicyclo[5.4.0]undec-7-ene at 120℃; under 8274.59 - 10136.4 Torr; | 88 %Spectr. |
| With palladium diacetate; potassium carbonate at 60℃; under 20521.4 Torr; for 12h; Green chemistry; chemoselective reaction; | 21 %Spectr. |

-
-
626-55-1
3-Bromopyridine

-
-
64-17-5
ethanol

-
-
201230-82-2
carbon monoxide

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine at 70℃; under 760.051 Torr; for 3h; Catalytic behavior; | 98% |


| Conditions | Yield |
|---|---|
| With sulfuric acid In toluene at 55℃; for 4h; | 97.2% |
| With sulfuric acid for 10h; Reflux; Sealed tube; | 83% |
| With sulfuric acid for 4h; Reflux; | 81% |

| Conditions | Yield |
|---|---|
| With cesium fluoride In acetonitrile for 2h; Heating; | 92% |
-
-
1701-72-0
1-(pyridin-3-yl)pentan-1-one

-
-
383-63-1
ethyl trifluoroacetate,

-
A
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
B
-
360-34-9
1,1,1-trifluoro-2-hexanone

| Conditions | Yield |
|---|---|
| Stage #1: ethyl trifluoroacetate, With sodium hydride In tetrahydrofuran at 20℃; for 0.166667h; Inert atmosphere; Schlenk technique; Stage #2: 1-(pyridin-3-yl)pentan-1-one In tetrahydrofuran at 0℃; for 2.5h; Inert atmosphere; Schlenk technique; Reflux; Stage #3: With hydrogenchloride In tetrahydrofuran; water at 0℃; for 0.25h; Inert atmosphere; Schlenk technique; | A 92% B n/a |

-
-
15226-74-1, 61091-28-9, 61117-58-6
dicobalt octacarbonyl

-
-
64-17-5
ethanol

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate at 50℃; for 12h; Schlenk technique; Inert atmosphere; chemoselective reaction; | 91% |

-
-
15226-74-1, 61091-28-9, 61117-58-6
dicobalt octacarbonyl

-
-
64-17-5
ethanol

-
-
107658-27-5
pyridin-3-yl trifluoromethanesulfonate

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2; potassium acetate at 50℃; for 12h; Schlenk technique; Inert atmosphere; chemoselective reaction; | 90% |

-
-
1120-90-7
3-iodopyridine

-
-
64-17-5
ethanol

-
-
13939-06-5, 199620-15-0
molybdenum hexacarbonyl

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With C35H20F34NO3(1-)*Pd(2+)*Cl(1-); N-ethyl-N,N-diisopropylamine In neat (no solvent) at 130℃; for 0.333333h; Microwave irradiation; | 89% |

-
-
14906-63-9
1-oxy-nicotinic acid ethyl ester

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With cerium(III) chloride heptahydrate; zinc In methanol at 20℃; for 2.5h; | 87% |

| Conditions | Yield |
|---|---|
| microwave irradiation; | 86% |
| In toluene at 110℃; for 24h; | 81% |

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane at 40℃; for 5h; | 86% |
| With chloro-trimethyl-silane at 20 - 40℃; for 5h; Inert atmosphere; | 86% |
-
-
626-60-8
3-Chloropyridine

-
-
15226-74-1, 61091-28-9, 61117-58-6
dicobalt octacarbonyl

-
-
64-17-5
ethanol

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With (S)-1-[(R)-2-(di-1-naphthylphosphino)ferrocenyl]ethyl-di-tert.-butylphosphine; potassium acetate; palladium diacetate at 80℃; for 12h; Schlenk technique; Inert atmosphere; chemoselective reaction; | 86% |

-
-
74-96-4
ethyl bromide

-
-
16518-17-5
potassium nicotinate

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide for 43h; Ambient temperature; | 82% |
-
-
626-55-1
3-Bromopyridine

-
-
1906-57-6
potassium monoethyl oxalate

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With palladium(II) trifluoroacetate; 1,3-bis-(diphenylphosphino)propane In 1-methyl-pyrrolidin-2-one at 150℃; for 16h; Inert atmosphere; | 82% |
-
-
59-67-6
nicotinic acid

-
-
109-63-7
boron trifluoride diethyl etherate

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| at 120℃; | 82% |
-
-
2017-01-8
tetraethoxy tellurium(IV)

-
-
98-92-0
nicotinamide

-
A
-
100-54-9
pyridine-3-carbonitrile

-
B
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In tetrachloromethane for 18h; Heating; | A 8% B 79% |

-
-
98-92-0
nicotinamide

-
A
-
100-54-9
pyridine-3-carbonitrile

-
B
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With tetraethoxy tellurium(IV) In tetrachloromethane for 18h; Heating; | A 8% B 79% |
-
-
1120-90-7
3-iodopyridine

-
-
1906-57-6
potassium monoethyl oxalate

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With palladium diacetate In 1-methyl-pyrrolidin-2-one at 20 - 140℃; for 24h; Inert atmosphere; | 79% |
-
-
626-55-1
3-Bromopyridine

-
-
15226-74-1, 61091-28-9, 61117-58-6
dicobalt octacarbonyl

-
-
64-17-5
ethanol

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; palladium diacetate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In toluene at 90℃; for 0.5h; Microwave irradiation; | 78% |


| Conditions | Yield |
|---|---|
| With 1,3-diazido-propane In neat (no solvent) at 20℃; for 0.333333h; | 78% |
-
-
151917-39-4
ethyl 6-iodonicotinate

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine; phenylsilane; indium(III) acetate In ethanol at 20℃; for 18h; | 75% |
| With indium In water for 10h; Heating; | 64% |
| With indium; water for 10h; Reflux; | 64% |
-
-
383-63-1
ethyl trifluoroacetate,

-
-
1327133-50-5
C12H11NOS

-
A
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
B
-
19305-01-2
1,1,1-trifluoro-4-(2-thiophenyl)butan-2-one

| Conditions | Yield |
|---|---|
| Stage #1: ethyl trifluoroacetate, With sodium hydride In tetrahydrofuran at 20℃; for 0.166667h; Inert atmosphere; Schlenk technique; Stage #2: C12H11NOS In tetrahydrofuran at 0℃; Inert atmosphere; Schlenk technique; Stage #3: With hydrogenchloride In tetrahydrofuran; water at 0℃; for 0.25h; Inert atmosphere; Schlenk technique; | A 75% B 74% |

-
-
110-89-4
piperidine

-
-
626-60-8
3-Chloropyridine

-
-
201230-82-2
carbon monoxide

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With 1,3-bis(dicyclohexylphosphino)propane bis(tetrafluoroborate) salt; sodium phenoxide; palladium diacetate In toluene at 100℃; under 760.051 Torr; for 16h; | 71% |

-
-
626-60-8
3-Chloropyridine

-
-
64-17-5
ethanol

-
-
201230-82-2
carbon monoxide

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With bis(benzonitrile)palladium(II) dichloride; sodium acetate; 1,4-di(diphenylphosphino)-butane at 100℃; under 750.075 Torr; for 16h; | 71% |


| Conditions | Yield |
|---|---|
| With Josiphos Et2P-Fc-PtBu2 ligand; sodium acetate; palladium diacetate at 130 - 135℃; under 2068.59 Torr; for 20h; | 70% |

-
-
109-97-7
pyrrole

-
-
22736-43-2
ethyl 2-bromo-2-diazoacetate

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sodium dithionite; c-type cytochrome maquette, C45 In ethanol at 5℃; for 2h; pH=8.6; Sealed tube; Inert atmosphere; Enzymatic reaction; | 69.4% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate for 144h; Heating; | 67% |
| With iron(III) chloride for 21h; Heating; | 63.3% |
-
-
626-55-1
3-Bromopyridine

-
-
95-92-1
oxalic acid diethyl ester

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With dmap; bis-triphenylphosphine-palladium(II) chloride In ethanol at 140℃; for 0.333333h; Microwave irradiation; | 66% |
-
-
2450-71-7
Propargylamine

-
-
623-47-2
propynoic acid ethyl ester

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; silver trifluoromethanesulfonate In toluene at 80℃; for 6h; Sealed tube; regioselective reaction; | 63% |
-
-
500-22-1
3-pyridinecarboxaldehyde

-
-
64-17-5
ethanol

-
A
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
B
-
35779-39-6
1,2-di(pyridine-3-yl)ethane-1,2-dione

| Conditions | Yield |
|---|---|
| With oxygen; Thiamine hydrochloride; triethylamine for 2h; Reflux; | A 30% B 58% |

-
-
96-20-8, 13054-87-0
2-aminobutanol

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
215120-51-7
N-(1-hydroxybutan-2-yl)nicotinamide

| Conditions | Yield |
|---|---|
| at 50℃; for 0.5h; | 100% |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
78-96-6, 1674-56-2
3-amino-2-propanol

-
-
122048-21-9
N-(2-hydroxypropyl)-3-pyridinecarboxamide

| Conditions | Yield |
|---|---|
| at 50℃; for 0.5h; | 100% |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
156-87-6
propan-1-ol-3-amine

-
-
53676-54-3
N-(3-hydroxypropyl)nicotinamide

| Conditions | Yield |
|---|---|
| at 50℃; for 0.5h; | 100% |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
181058-08-2
1,3,5-tris(bromomethyl)-2,4,6-triethylbenzene

| Conditions | Yield |
|---|---|
| In acetonitrile for 6h; Heating; | 100% |

| Conditions | Yield |
|---|---|
| at 80 - 85℃; for 6.5h; | 99.5% |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
71962-74-8
Ethyl nipecotate

| Conditions | Yield |
|---|---|
| With hydrogen In hexane at 100℃; under 7600.51 Torr; for 24h; Temperature; Pressure; Autoclave; | 99% |
| With sodium hydroxide; hydrogen; nickel In ethanol; water at 20 - 40℃; Electrolysis; | 98% |
| With 1,4-dioxane; nickel at 165℃; under 110326 - 220652 Torr; Hydrogenation; |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
100-39-0
benzyl bromide

-
-
72551-50-9
N-benzyl-3-(ethoxycarbonyl)pyridinium bromide

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 18h; | 98% |
| In isopropyl alcohol at 20℃; for 18h; | 90% |
| In nitrobenzene at 40℃; Rate constant; | |
| In acetone Reflux; |

| Conditions | Yield |
|---|---|
| With PPA at 155℃; for 4.5h; | 98% |
| With alkaline H2O In dimethyl sulfoxide at 30℃; Rate constant; other solvent (EtOH); variation of water concentrations; | |
| esterase in WBN; IL-Ht rat abdominal skin homogenate at 37℃; Enzyme kinetics; |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
86931-32-0
N-(N,N-diethylaminomethyl)-2-piperidone

| Conditions | Yield |
|---|---|
| With sodium hydride In toluene for 8h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With n-butyllithium; diisobutylaluminium hydride; tert-butyl alcohol In tetrahydrofuran; hexane at 0℃; | 97% |
| Multi-step reaction with 2 steps 1: diethyl ether; lithium alanate 2: lead (IV)-acetate; benzene View Scheme |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
141-43-5
ethanolamine

-
-
6265-73-2
N-(2-hydroxyethyl)nicotinamide

| Conditions | Yield |
|---|---|
| Stage #1: 3-pyridinecarboxylic acid ethyl ester; ethanolamine at 125℃; for 4h; Large scale; Stage #2: With acetone at 45 - 60℃; for 1h; Large scale; Stage #3: at 10 - 30℃; for 2.5h; Temperature; Large scale; | 96.6% |
| 64.5% | |
| 64.5% |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
50-01-1
guanidine hydrochloride

-
-
6531-75-5
N-(pyridyl-3-carbonyl)guanidine

| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol for 12h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 80℃; for 36h; | 95% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran dry Ar-flushed Schlenk flask charged with organic compound, soln. of base added dropwise, stirred at -78 °C for 15 min; | 95% |


| Conditions | Yield |
|---|---|
| Stage #1: 3-pyridinecarboxylic acid ethyl ester; Allyl chloroformate In tetrahydrofuran at -20℃; for 0.166667h; Molecular sieve; Stage #2: C10H10BrN3O With bis(η3-allyl-μ-chloropalladium(II)) In tetrahydrofuran at -20℃; for 13h; diastereoselective reaction; | 95% |


| Conditions | Yield |
|---|---|
| Stage #1: 3-pyridinecarboxylic acid ethyl ester With potassium phosphate; chloro(1,5-cyclooctadiene)rhodium(I) dimer; P,P'-(1,2-ethanediyl)bis{bis[3,5-di(trifluoromethyl)phenyl]phosphane} In toluene for 0.0833333h; Inert atmosphere; Microwave irradiation; Stage #2: methyl methacrylate In toluene at 120℃; for 24h; Inert atmosphere; Microwave irradiation; Sealed tube; regioselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate monohydrate In methanol at 140℃; for 1h; Microwave irradiation; Sealed tube; | 93% |
| With hydrazine hydrate In ethanol for 1.08333h; Ambient temperature; | 85% |
| With hydrazine hydrate In ethanol Reflux; Sealed tube; | 85% |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
100422-07-9
2,2,2-trifluoroethyl(phenyl)iodonium trifluoromethanesulfonate

-
-
135654-45-4
N-(2,2,2-Trifluoroethyl)-3-(ethoxycarbonyl)pyridinium triflate

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.166667h; Ambient temperature; | 93% |

| Conditions | Yield |
|---|---|
| With graphene oxide In neat (no solvent) at 100℃; for 18h; Sealed tube; | 92% |
| With silica gel In neat (no solvent) at 100℃; for 12h; Inert atmosphere; Sealed tube; | 87% |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
36016-40-7
mesitylenesulfonylhydroxylamine

-
-
40146-54-1
2,4,6-Trimethyl-benzenesulfonate1-amino-3-ethoxycarbonyl-pyridinium;

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.5h; Ambient temperature; | 92% |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
1788-95-0
3-(4-bromophenyl)-2-methylquinazolin-4(3H)-one

-
-
73283-34-8
2-[2-Oxo-2-(3-pyridyl)ethyl]-3-p-bromophenyl-4(3H)-quinazolinone

| Conditions | Yield |
|---|---|
| With sodium hydride In 1,2-dimethoxyethane Heating; | 92% |
| 2.43 g (92%) |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
108-24-7
acetic anhydride

-
-
917112-54-0
ethyl 1-acetyl-1,4,5,6-tetrahydropyridine-3-carboxylate

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal under 5171.48 Torr; | 92% |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
141-78-6
ethyl acetate

-
A
-
6283-81-4
3-oxo-3-pyridin-3-yl-propionic acid ethyl ester

-
B
-
141-97-9
ethyl acetoacetate

| Conditions | Yield |
|---|---|
| With lithium hexamethyldisilazane In tetrahydrofuran at -40℃; for 0.333333h; Claisen condensation; Inert atmosphere; | A 92% B n/a |

-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In acetonitrile for 60h; Inert atmosphere; Reflux; | 92% |
-
-
614-18-6
3-pyridinecarboxylic acid ethyl ester

-
-
74-88-4
methyl iodide

-
-
10129-58-5
3-(ethoxycarbonyl)-1-methylpyridin-1-ium iodide

| Conditions | Yield |
|---|---|
| In nitromethane for 12h; Ambient temperature; | 91% |
| In isopropyl alcohol for 18h; | 87% |
| In nitrobenzene at 35℃; Rate constant; |
Ethyl nicotinoate Chemical Properties
Molecular Structure of Ethyl nicotinoate (CAS NO.614-18-6):
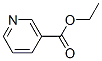
IUPAC Name:ethyl pyridine-3-carboxylate
Molecular Formula: C8H9NO2
Molecular Weight: 151.162560 g/mol
Melting Point: 8-10 °C(lit.)
H bond acceptors: 3
H bond donors: 0
Freely Rotating Bonds: 3
Polar Surface Area: 39.19 Å2
Index of Refraction: 1.506
Molar Refractivity: 40.75 cm3
Molar Volume: 137 cm3
Surface Tension: 40.6 dyne/cm
Density: 1.102 g/cm3
Flash Point: 93.3 °C
Enthalpy of Vaporization: 46.05 kJ/mol
Boiling Point: 224 °C at 760 mmHg
Vapour Pressure: 0.0934 mmHg at 25°C
Storage temp: 2-8°C
Water Solubility: miscible
Chemical Properties: clear colourless to yellow liquid
Synonyms of Ethyl nicotinoate(CAS NO.614-18-6): nicotinic acid ethyl ester ; ethyl 3-pyridinecarboxylate ; ethyl pyridine-3-carboxylate ; ethyl nicotinate ; ethyl nicotinoate ; 3-pyridinecarboxylic acid ethyl ester ; 3-(ethoxycarbonyl)pyridine
Ethyl nicotinoate Safety Profile
Safety Information of Ethyl nicotinoate(CAS NO.614-18-6):
Hazard Codes:  Xi
Xi
Risk Statements: 37/38-41-36/38-36/37/38
37/38:Irritating to respiratory system and skin
41:Risk of serious damage to eyes
36/38:Irritating to eyes and skin
36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements: 26-39-37/39-36
26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
39:Wear eye/face protection
37/39:Wear suitable gloves and eye/face protection
36:Wear suitable protective clothing
Ethyl nicotinoate Specification
Ethyl nicotinoate (CAS NO.614-18-6) should be stored in a cool and a tightly closed container, can be used in organic synthesis.
Related Products
- Ethyl (2,4,6-trimethylbenzoyl) phenylphosphinate
- Ethyl (2-amino-4-hydroxy-6-methyl-5-pyrimidinyl)acetate
- Ethyl (3R)-piperidine-3-carboxylate
- Ethyl (3R,S)-2,2-difluoro-3-hydroxy-3-(2,2-dimethyldioxolan-4-yl)propionate
- Ethyl (3S)-4-bromo-3-hydroxybutanoate
- Ethyl (3S)-piperidine-3-carboxylate
- Ethyl (3-trifluoromethylbenzoyl)acetate
- Ethyl (3α,5β,7α,12α)-3,7,12-trihydroxycholan-24-oate
- Ethyl (6-amino-9H-purin-9-yl)acetate
- Ethyl (6-aminopyridin-2-yl)acetate
- 61419-02-1
- 61419-61-2
- 614-19-7
- 6141-98-6
- 614-20-0
- 61420-92-6
- 614-21-1
- 61422-45-5
- 6142-30-9
- 614-23-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View