-
Name
Ginkgolide B
- EINECS 604-876-0
- CAS No. 15291-77-7
- Article Data5
- CAS DataBase
- Density 1.645 g/cm3
- Solubility Soluble in DMSO. Slightly soluble in water and ethanol
- Melting Point 280 °C (dec.)
- Formula C20H24O10
- Boiling Point 762.416 °C at 760 mmHg
- Molecular Weight 424.405
- Flash Point 274.337 °C
- Transport Information
- Appearance white crystal powder
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms GinkgolideA, 1-hydroxy-, (1b)- (8CI);5H-Dicyclopenta[b,c]furan-3,5a(6H)-diacetic acid, 6-tert-butyl-3a-carboxyhexahydro-a5a,1,2,3,5,8-hexahydroxy-a3-methyl-, tri-g-lactone (8CI);9H-1,7a-(Epoxymethano)-1H,6aH-cyclopenta[c]furo[2,3-b]furo[3',2':3,4]cyclopenta[1,2-d]furan-5,9,12(4H)-trione,3-(1,1-dimethylethyl)hexahydro-4,7b,11-trihydroxy-8-methyl-, [1R-(1a,3b,3aS*,4b,6aa,7aa,7ba,8a,10aa,11b,11aR*)]-;BN 52051;9H-1,7a-(Epoxymethano)-1H,6aH-cyclopenta[c]furo[2,3-b]furo[3',2':3,4]cyclopenta[1,2-d]furan-5,9,12(4H)-trione,3-(1,1-dimethylethyl)hexahydro-4,7b,11-trihydroxy-8-methyl-, (1R,3S,3aS,4R,6aR,7aR,7bR,8S,10aS,11R,11aR)-;
- PSA 148.82000
- LogP -1.36950
Synthetic route
-
-
170288-58-1
α-benzylginkgolide B

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol under 2888 Torr; for 24h; | 97% |
-
-
130523-06-7
1-O-(tert-Butyldiphenylsilyl)ginkgolid B

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 2h; | 73% |
-
-
145473-36-5
C28H32O11

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In methanol at 23℃; under 760 Torr; for 5h; Yield given; |
-
-
173102-79-9
7-trifluoromethanesulfonyloxy ginkgolide B

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| With tetrabutylammonium borohydride |
-
-
15291-76-6
1,3,7,10-Tetrahydroxyginkgolid

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: pyridine 2: nBu4NBH4 View Scheme |
-
-
15291-76-6, 119322-55-3
gingkolide C

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: diisopropylethylamine / acetonitrile / 5 h / 0 °C 2: 63 percent / 4-N,N-dimethylaminopyridine / acetonitrile / 12 h / 5 °C 3: tri-n-butyltin hydride, bisazoisobutyronitrile / benzene / 12 h / Heating 4: H2 / Pd-C / methanol / 5 h / 23 °C / 760 Torr View Scheme |
-
-
145497-37-6
C28H32O12

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 63 percent / 4-N,N-dimethylaminopyridine / acetonitrile / 12 h / 5 °C 2: tri-n-butyltin hydride, bisazoisobutyronitrile / benzene / 12 h / Heating 3: H2 / Pd-C / methanol / 5 h / 23 °C / 760 Torr View Scheme |
-
-
145497-38-7
C35H31F5O13S

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tri-n-butyltin hydride, bisazoisobutyronitrile / benzene / 12 h / Heating 2: H2 / Pd-C / methanol / 5 h / 23 °C / 760 Torr View Scheme |
-
-
15291-76-6
Ginkgolide C

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 95 percent / imidazole / dimethylformamide / 48 h 2: 66 percent / DMAP / acetonitrile / 24 h 3: 93 percent / tributyltin hydride, AIBN / toluene / 3 h / Heating 4: 73 percent / tetrabutylammonium fluoride / tetrahydrofuran / 2 h View Scheme |
-
-
130523-04-5
1-O-(tert-Butyldiphenylsilyl)ginkgolid C

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 66 percent / DMAP / acetonitrile / 24 h 2: 93 percent / tributyltin hydride, AIBN / toluene / 3 h / Heating 3: 73 percent / tetrabutylammonium fluoride / tetrahydrofuran / 2 h View Scheme |
-
-
130523-05-6
1-O-(tert-Butyldiphenylsilyl)-7-O-(phenyloxythiocarbonyl)ginkgolid C

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 93 percent / tributyltin hydride, AIBN / toluene / 3 h / Heating 2: 73 percent / tetrabutylammonium fluoride / tetrahydrofuran / 2 h View Scheme |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 0.5h; Alkylation; Heating; | 98.3% |
-
-
15291-77-7
ginkgolide B

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
130523-06-7
1-(tert-butyldiphenylsilyloxy)-3,10-dihydroxyginkgolid

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide for 48h; Ambient temperature; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 0.5h; Alkylation; Heating; | 96.7% |
-
-
4584-46-7
(2-chloroethyl)dimethylamine hydrochloride

-
-
15291-77-7
ginkgolide B

-
-
914251-20-0
10-O-(2-dimethylaminoethyl)ginkgolide B

| Conditions | Yield |
|---|---|
| With calcium carbonate; potassium iodide In water; acetonitrile at 85℃; Reagent/catalyst; Temperature; Flow reactor; | 94.1% |
| With calcium carbonate; potassium iodide In water; acetonitrile at 85℃; for 0.05h; Reagent/catalyst; Temperature; Concentration; Flow reactor; | 94.1% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 0.5h; Alkylation; Heating; | 92.3% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 0.5h; Alkylation; Heating; | 90.1% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 0.5h; Acylation; Heating; | 89.4% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 0.5h; Alkylation; Heating; | 89.2% |
-
-
18355-05-0
1-hydroxybenzotriazolyl acetate

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| With pyridine; dmap In N,N-dimethyl-formamide | A 88% B 9% |


| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 0.5h; Alkylation; Heating; | 87.8% |
-
-
4535-90-4
N-methyl-2-chloroethylamine hydrochloride

-
-
15291-77-7
ginkgolide B

-
-
914251-23-3
10-O-methylaminoethyl ginkgolide B

| Conditions | Yield |
|---|---|
| With calcium carbonate; potassium iodide In water; acetonitrile at 85℃; Flow reactor; | 83.5% |

| Conditions | Yield |
|---|---|
| With calcium carbonate; potassium iodide In water; acetonitrile at 85℃; Flow reactor; | 79.4% |
-
-
15291-77-7
ginkgolide B

-
-
105-36-2
ethyl bromoacetate

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 0.5h; Alkylation; Heating; | A 78.3% B 12.1% |


| Conditions | Yield |
|---|---|
| With calcium carbonate; potassium iodide In water; acetonitrile at 85℃; Flow reactor; | 76.3% |
| With calcium carbonate; potassium iodide In water; acetonitrile at 85℃; for 0.05h; Flow reactor; | 76.3% |

| Conditions | Yield |
|---|---|
| With triethylamine; potassium iodide In chloroform Reflux; | 75% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile at 75℃; for 0.75h; | 72.9% |
-
-
16004-15-2
1-bromomethyl-4-iodobenzene

-
-
15291-77-7
ginkgolide B

-
-
170289-24-4
10-O-p-iodobenzylginkgolide B

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile at 70℃; for 0.666667h; | 72.4% |

| Conditions | Yield |
|---|---|
| With triethylamine; potassium iodide In chloroform Reflux; | 71% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 0.5h; Alkylation; Heating; | 65.3% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 24h; Reflux; Inert atmosphere; | 65% |
| With potassium carbonate; potassium iodide In acetonitrile at 100℃; for 2.5h; |
-
-
15291-77-7
ginkgolide B

-
-
589-15-1
1-bromomethyl-4-bromobenzene

-
-
170289-51-7
10-O-p-bromobenzylginkgolide B

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile at 70℃; for 0.833333h; | 64.5% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic acid In tetrahydrofuran for 8h; Condensation; Heating; | 61.8% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 6h; | 61.25% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetonitrile for 0.5h; Alkylation; Heating; | A 26.7% B 60.1% |

-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In tetrahydrofuran for 2h; Reflux; | 58% |
-
-
15291-77-7
ginkgolide B

-
-
84424-42-0
methyl 2-(2-iodoethoxy)acetate

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In tetrahydrofuran for 2h; Reflux; | 58% |

-
-
15291-77-7
ginkgolide B

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In tetrahydrofuran for 2h; Reflux; | 56% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 6h; | A 8.4% B 55.57% |

Ginkgolide B Specification
The Ginkgolide B, with the CAS registry number 15291-77-7, is also known as 9H-1,7a-(Epoxymethano)-1H,6aH-cyclopenta[c]furo[2,3-b]furo[3',2':3,4]cyclopenta[1,2-d]furan-5,9,12(4H)-trione,3-(1,1-dimethylethyl)hexahydro-4,7b,11-trihydroxy-8-methyl-, (1R,3S,3aS,4R,6aR,7aR,7bR,8S,10aS,11R,11aR)-. It belongs to the product categories of Saponins; Intermediates & Fine Chemicals; Pharmaceuticals; PAF receptor. This chemical's molecular formula is C20H24O10 and molecular weight is 424.40. Its classification codes are: (1)Cardiovascular Agents; (2)Fibrin Modulating Agents; (3)Fibrinolytic agents; (4)Hematologic Agents. It is a PAF-receptor antagonist. It may be effective as preventive treatment in reducing migraine attack frequency.
Physical properties of Ginkgolide B are: (1)ACD/LogP: 2.07; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 2.07; (4)ACD/LogD (pH 7.4): 2.07; (5)ACD/BCF (pH 5.5): 22.036; (6)ACD/BCF (pH 7.4): 22.03; (7)ACD/KOC (pH 5.5): 318.44; (8)ACD/KOC (pH 7.4): 318.362; (9)#H bond acceptors: 10; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 148.82 Å2; (13)Index of Refraction: 1.651; (14)Molar Refractivity: 94.203 cm3; (15)Molar Volume: 258.046 cm3; (16)Polarizability: 37.345×10-24cm3; (17)Surface Tension: 79 dyne/cm; (18)Density: 1.645 g/cm3; (19)Flash Point: 274.337 °C; (20)Enthalpy of Vaporization: 126.648 kJ/mol; (21)Boiling Point: 762.416 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
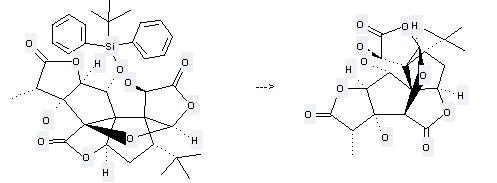
Preparation: this chemical can be prepared by 1-O-(tert-Butyldiphenylsilyl)ginkgolid B. This reaction will need reagent tetrabutylammonium fluoride and solvent tetrahydrofuran with the reaction time of 2 hours. The yield is about 73%.
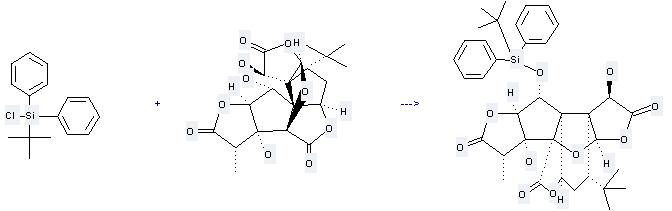
Uses of Ginkgolide B: it can be used to produce 1-(tert-butyldiphenylsilyloxy)-3,10-dihydroxyginkgolid at the ambient temperature. It will need reagent imidazole and solvent dimethylformamide with the reaction time of 48 hours. The yield is about 98%.
You can still convert the following datas into molecular structure:
(1)SMILES: C[C@H]1[C@@]2([C@]34O[C@@H]5OC(=O)[C@@H]([C@]56C3([C@@H](C[C@H]6[C@](C)(C)C)OC4=O)[C@H](C2OC1=O)O)O)O
(2)Std. InChI: InChI=1S/C20H24O10/c1-6-12(23)28-11-9(21)18-8-5-7(16(2,3)4)17(18)10(22)13(24)29-15(17)30-20(18,14(25)27-8)19(6,11)26/h6-11,15,21-22,26H,5H2,1-4H3/t6-,7+,8-,9+,10+,11?,15+,17+,18?,19-,20-/m1/s1
(3)Std. InChIKey: SQOJOAFXDQDRGF-REVVDZANSA-N
Related Products
- Ginkgolide A
- Ginkgolide B
- Ginkgolide C
- Ginkgolide J
- Ginkgolide M
- 15291-78-8
- 152918-47-3
- 152922-71-9
- 152922-73-1
- 152929-67-4
- 15293-77-3
- 152940-76-6
- 1529-41-5
- 152946-68-4
- 1529-47-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View