-
Name
Isotretinoin
- EINECS 225-296-0
- CAS No. 4759-48-2
- Article Data68
- CAS DataBase
- Density 1.011 g/cm3
- Solubility insoluble in water
- Melting Point 172-175 °C(lit.)
- Formula C20H28O2
- Boiling Point 462.8 °C at 760 mmHg
- Molecular Weight 300.441
- Flash Point 350.6 °C
- Transport Information
- Appearance yellowish crystalline powder
- Safety 53-26-36/37/39-45
- Risk Codes 61-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms Isotretinoin [USAN:BAN:INN];(2Z,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethyl-1-cyclohexenyl)nona-2,4,6,8-tetraenoate;Retinoic acid, (13cis)-;13-cis-Retinoic acid;Isotretinoin Retinoic acid;Prestwick_642;Neovitamin A acid;Claravis;Teriosal;Roaccutan;Isotretion;Isotretinoin?;Isotretinoine [INN-French];isoretinoin;4-09-00-02388 (Beilstein Handbook Reference);
- PSA 37.30000
- LogP 5.60260
Synthetic route
-
-
20013-34-7
acide-2-carboxy-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2E,4E,6E,8E-tetraenoique

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine for 2h; Heating; | 90% |
| With lutidine Heating; | 63% |
| With 2,6-dimethylpyridine for 1.5h; Decarboxylation; | 56% |
| With 2,6-dimethylpyridine for 0.3h; Heating; |
-
-
70143-04-3
(Z)-3-methyl-4-oxobut-2-enoic acid

-
-
62285-98-7
5-(2,6,6-trimethylcyclohexenyl)-3-methyl-2,4-pentadienyltriphenylphosphonium bromide

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| Stage #1: (Z)-3-methyl-4-oxobut-2-enoic acid; 5-(2,6,6-trimethylcyclohexenyl)-3-methyl-2,4-pentadienyltriphenylphosphonium bromide With sodium hydroxide In isopropyl alcohol at 20 - 30℃; for 3h; Wittig Olefination; Inert atmosphere; Stage #2: With hydrogenchloride; palladium diacetate In isopropyl alcohol at 60℃; pH=7 - 8; | 86.3% |
| Stage #1: (Z)-3-methyl-4-oxobut-2-enoic acid; 5-(2,6,6-trimethylcyclohexenyl)-3-methyl-2,4-pentadienyltriphenylphosphonium bromide With sodium hydroxide In isopropyl alcohol at 20 - 30℃; for 3h; Wittig Olefination; Inert atmosphere; Stage #2: With hydrogenchloride; palladium diacetate In isopropyl alcohol at 60℃; pH=7-8; Inert atmosphere; | 86.3% |
-
-
43059-50-3
4-methyl-6-[(1E,3E)-2-methyl-4-(2,6,6-trimethylcyclohex-1-en-1-yl)buta-1,3-dien-1-yl]-5,6-dihydro-2H-pyran-2-one

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 0℃; for 1h; | 80% |
| With pyrographite for 0.5h; Reflux; | 55 g |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 0.5h; Heating; Yield given; | A n/a B 77% |
| With potassium hydroxide In ethanol for 0.5h; Heating; Yields of byproduct given; | A n/a B 77% |
-
-
59699-82-0
13-cis-ethyl retinoate

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 50℃; | 70% |
| With sodium hydroxide |
-
-
220212-07-7
(2Z,4E)-5-iodo-3-methylpenta-2,4-dienoic acid

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| [PdCl2(NCMe)2] In N,N-dimethyl-formamide at 25℃; for 3h; Stille cross-coupling reaction; | 70% |
-
-
81176-73-0
11-cis,13-cis-12-carboxyretinoic acid

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| With copper (I) acetate In various solvent(s) at 110℃; for 2.5h; | 68.2% |
| With 2,4-lutidine; copper diacetate |
-
-
105593-28-0
β-ionone

-
-
75-16-1
methylmagnesium bromide

-
-
419550-09-7
dimethyl 2-[(2E)-3-(dimethylamino)-1-methyl-2-propenylidene]malonate

-
A
-
4759-48-2
13-cis-retinoic acid

-
B
-
302-79-4
all-trans-retinoic-acid

| Conditions | Yield |
|---|---|
| Multistep reaction.; | A 12% B 61% |

-
-
20013-34-7
acide-2-carboxy-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2E,4E,6E,8E-tetraenoique

-
A
-
4759-48-2
13-cis-retinoic acid

-
B
-
302-79-4
all-trans-retinoic-acid

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; for 20h; | A n/a B 60% |
| With barium dihydroxide In benzene for 0.5h; |
-
-
81176-73-0
11-cis,13-cis-12-carboxyretinoic acid

-
A
-
4759-48-2
13-cis-retinoic acid

-
B
-
70244-78-9, 94440-28-5
2-((1E,3E,5E)-3,7-dimethylocta-1,3,5,7-tetraen-1-yl)-1,3,3-trimethylcyclohex-1-ene

| Conditions | Yield |
|---|---|
| With copper (I) acetate In pyridine at 100℃; for 2h; Product distribution; other solvents, other temperatures, other times; | A 49.8% B 33.9% |
| With copper (I) acetate In pyridine at 100℃; for 3h; | A 45.6% B 30.8% |
-
-
87424-83-7
4-Methyl-6-<(1E,3E)-2-methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1,3-butadien-1-yl>-2H-pyran-2-on

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; sodium tetrahydroborate In methanol for 6h; Heating; | 42% |
-
-
1620084-74-3
(2E,4E,6E,8E)-3,7-dimethyl-9-{6,6-dimethyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolanyl)-1-cyclohexenyl}-2,4,6,8-nonatetraenoicacid ethyl ester

-
-
74-88-4
methyl iodide

-
A
-
4759-48-2
13-cis-retinoic acid


-
C
-
302-79-4
all-trans-retinoic-acid

| Conditions | Yield |
|---|---|
| Stage #1: (2E,4E,6E,8E)-3,7-dimethyl-9-{6,6-dimethyl-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolanyl)-1-cyclohexenyl}-2,4,6,8-nonatetraenoicacid ethyl ester; methyl iodide With tris-(dibenzylideneacetone)dipalladium(0); potassium carbonate; tris-(o-tolyl)phosphine In water; N,N-dimethyl-formamide at 60℃; for 0.0833333h; Inert atmosphere; Schlenk technique; Stage #2: With potassium hydroxide In methanol; water; N,N-dimethyl-formamide at 100℃; for 0.0333333h; Inert atmosphere; Schlenk technique; Stage #3: With formic acid In methanol; water; N,N-dimethyl-formamide Inert atmosphere; Schlenk technique; | A 10% B 0.5% C 32% |

-
-
638-10-8
ethyl 3-methylbut-2-enoate

-
-
3917-41-7
(2E,4E)-3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienal

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| With sodium amide |
-
-
41551-56-8, 78088-24-1, 78088-25-2, 78088-26-3
methyl 5-benzenesulphonyl-3.7-dimethyl-9-(2'.6'.6'.trimethyl-cyclohexen-1'-yl)-2.6.8-nonatrienoate

-
A
-
4759-48-2
13-cis-retinoic acid

-
B
-
302-79-4
all-trans-retinoic-acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 2.5h; Heating; Yield given. Yields of byproduct given; |
-
-
146558-55-6
8-<2-(methylthio)-1,3-dithian-2-yl>-3,7-dimethyl-1-(2,6,6-trimethylcyclohex-1-enyl)octa-1E,3E,5E,7E-tetraene

-
A
-
4759-48-2
13-cis-retinoic acid

-
B
-
302-79-4
all-trans-retinoic-acid

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction. Yields of byproduct given; |
-
-
16760-45-5
(2Z,4E,6E,8E)-methyl 3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoate

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol at 50℃; for 2h; |
-
-
73395-75-2
(E)-2-(but-1-en-3-ynyl)-1,3,3-trimethylcyclohex-1-ene

-
-
220212-07-7
(2Z,4E)-5-iodo-3-methylpenta-2,4-dienoic acid

-
-
74-88-4
methyl iodide

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; |

-
-
472-86-6
13-cis-vitamin A aldehyde

-
A
-
4759-48-2
13-cis-retinoic acid

-
B
-
71423-68-2
4-hydroxy-13-cis-retinal

| Conditions | Yield |
|---|---|
| With rabbit liver microsomal P450 1A1 isozyme; NADPH; NADPH-cytochrome P450 reductase In phosphate buffer at 30℃; for 0.25h; pH=7.4; Enzyme kinetics; Further Variations:; Reaction partners; Oxidation; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With cytosolic aldehyde dehydrogenase from human liver In phosphate buffer; dimethyl sulfoxide at 25℃; for 1h; pH=7.5; Enzyme kinetics; | |
| Multi-step reaction with 2 steps 1: 60 percent / NaCN, AgO, MnO2 / 14 h / 40 °C 2: 70 percent / 6 M NaOH / ethanol / 50 °C View Scheme | |
| Multi-step reaction with 2 steps 1: AgO, MnO2, NaCN 2: NaOH View Scheme |
-
-
20013-34-7
acide-2-carboxy-3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2E,4E,6E,8E-tetraenoique

-
A
-
4759-48-2
13-cis-retinoic acid

-
B
-
302-79-4
all-trans-retinoic-acid

-
C
-
70244-78-9, 94440-28-5
2-((1E,3E,5E)-3,7-dimethylocta-1,3,5,7-tetraen-1-yl)-1,3,3-trimethylcyclohex-1-ene

| Conditions | Yield |
|---|---|
| With triethylamine In benzene for 0.3h; Heating; |


| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water at 40℃; for 0.75h; | A 1.05 g B 5 g |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / potassium hydroxide / methanol / 64 h / 20 °C 2: 49.8 percent / copper acetate / pyridine; various solvent(s) / 2 h / 100 °C / other solvents, other temperatures, other times View Scheme | |
| Multi-step reaction with 2 steps 1: 85 percent / potassium hydroxide / methanol / 64 h / 20 °C 2: 68.2 percent / copper acetate / various solvent(s) / 2.5 h / 110 °C View Scheme |
-
-
3917-41-7
(2E,4E)-3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienal

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 85 percent / potassium hydroxide / methanol / 64 h / 20 °C 2: 49.8 percent / copper acetate / pyridine; various solvent(s) / 2 h / 100 °C / other solvents, other temperatures, other times View Scheme | |
| Multi-step reaction with 2 steps 1: 85 percent / potassium hydroxide / methanol / 64 h / 20 °C 2: 68.2 percent / copper acetate / various solvent(s) / 2.5 h / 110 °C View Scheme | |
| Multi-step reaction with 3 steps 1: MeOK 2: KOH / methanol; H2O 3: 2,6-dimethylpiridine / 0.3 h / Heating View Scheme |
-
-
13830-91-6
diethyl bromoisopropylidenemalonate

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: BuLi / tetrahydrofuran; hexane / 0.5 h / -78 °C 1.2: 74 percent / tetrahydrofuran; hexane / 1.5 h / -78 - 20 °C 2.1: 58 percent / KOH / propan-2-ol / 27 h / 20 °C 3.1: 63 percent / lutidine / Heating View Scheme |
-
-
38987-91-6
5-(2,6,6-trimethyl-cyclohex-1-enyl)-3-methyl-1-phenyl-sulphonyl-penta-2,4-diene

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: BuLi / tetrahydrofuran; hexane / 0.5 h / -78 °C 1.2: 74 percent / tetrahydrofuran; hexane / 1.5 h / -78 - 20 °C 2.1: 58 percent / KOH / propan-2-ol / 27 h / 20 °C 3.1: 63 percent / lutidine / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: potassium t-butoxide / tetrahydrofuran; various solvent(s) / 1 h / -65 °C 2: potassium hydroxide / methanol / 2.5 h / Heating View Scheme |
-
-
777916-02-6
2-[(4E,6E)-3-Benzenesulfonyl-1,5-dimethyl-7-(2,6,6-trimethyl-cyclohex-1-enyl)-hepta-4,6-dienylidene]-malonic acid diethyl ester

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 58 percent / KOH / propan-2-ol / 27 h / 20 °C 2: 63 percent / lutidine / Heating View Scheme |
-
-
22035-53-6
dimethyl isopropylidenemalonate

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 95 percent / methanol / 18 h / 95 °C 2: 65 percent / iPr2NH / 1,2-dimethoxy-ethane; hexane / 2 h / Heating 3: 69 percent / tetrahydrofuran / 0.5 h / -10 °C 4: 1.05 g / KOH / ethanol; H2O / 0.75 h / 40 °C View Scheme | |
| Multi-step reaction with 3 steps 1: MeOK 2: KOH / methanol; H2O 3: 2,6-dimethylpiridine / 0.3 h / Heating View Scheme | |
| Multi-step reaction with 3 steps 1: MeOK 2: KOH / methanol; H2O 3: Ba(OH)2 / benzene / 0.5 h View Scheme |
-
-
419550-09-7
dimethyl 2-[(2E)-3-(dimethylamino)-1-methyl-2-propenylidene]malonate

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 65 percent / iPr2NH / 1,2-dimethoxy-ethane; hexane / 2 h / Heating 2: 69 percent / tetrahydrofuran / 0.5 h / -10 °C 3: 1.05 g / KOH / ethanol; H2O / 0.75 h / 40 °C View Scheme |
-
-
419550-36-0
dimethyl 2-[(3E,6E)-1-methyl-5-oxo-7-(2,6,6-trimethyl-1-cyclohexen-1-yl)-1,3,6-heptatrienyl]malonate

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 69 percent / tetrahydrofuran / 0.5 h / -10 °C 2: 1.05 g / KOH / ethanol; H2O / 0.75 h / 40 °C View Scheme |
-
-
4759-48-2
13-cis-retinoic acid

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
16760-45-5
(2Z,4E,6E,8E)-methyl 3,7-dimethyl-9-(2,6,6-trimethylcyclohex-1-en-1-yl)nona-2,4,6,8-tetraenoate

| Conditions | Yield |
|---|---|
| In methanol; hexane; benzene | 83% |
| In methanol; hexane; benzene | 83% |
| In methanol; hexane; benzene at 20℃; for 0.75h; |

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0℃; for 24h; optical yield given as %de; | 81% |
-
-
4759-48-2
13-cis-retinoic acid

-
-
53839-60-4
retinoyl chloride

-
-
138752-02-0
all-(E)-retinoic and 13-(Z)-retinoic anhydride

| Conditions | Yield |
|---|---|
| With triethylamine In benzene for 0.5h; Ambient temperature; | 76.7% |
-
-
4759-48-2
13-cis-retinoic acid

-
-
64291-41-4
(2R,3R,4S,5R,6S)-2-(acetoxymethyl)-6-(4-(hydroxymethyl)phenoxy)-tetrahydro-2H-pyran-3,4,5-triyl triacetate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; | 74% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 10h; | 73.2% |

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane for 5h; | 70% |
-
-
929016-73-9
acetic acid 4,5-diacetoxy-6-acetoxymethyl-2-(5-hydroxymethyl-2-methoxy-phenoxy)-tetrahydro-pyran-3-yl ester

-
-
4759-48-2
13-cis-retinoic acid

-
-
929016-74-0
3,7-dimethyl-9-(2,6,6-trimethyl-cyclohex-1-enyl)-nona-2,4,6,8-tetraenoic acid 4-methoxy-3-(3,4,5-triacetoxy-6-acetoxymethyl-tetrahydro-pyran-2-yloxy)-benzyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 10h; | 67.1% |

| Conditions | Yield |
|---|---|
| With 1,1'-carbonyldiimidazole In benzene 1.) 20-25 deg C, 3 h, 50 deg C, 15 min, 2.) 10-20 deg C, 2 h; | 66% |

| Conditions | Yield |
|---|---|
| With dmap; potassium hydroxide In dichloromethane for 9h; Heating; | 65% |

| Conditions | Yield |
|---|---|
| With dmap; potassium hydroxide In dichloromethane for 6h; Heating; | 61.4% |
-
-
13325-10-5
4-Aminobutanol

-
-
4759-48-2
13-cis-retinoic acid

-
-
84680-31-9
13-cis-N-(4-Hydroxybutyl)retinamide

| Conditions | Yield |
|---|---|
| With 1,1'-carbonyldiimidazole In benzene 1.) 20-25 deg C, 3 h, 50 deg C, 15 min, 2.) 10-20 deg C, 2 h; | 60% |

| Conditions | Yield |
|---|---|
| With dmap; potassium hydroxide In dichloromethane for 9h; Heating; | 55.6% |
-
-
929016-65-9
[2-(hydroxymethyl)phenyl]-2,3,4,6-tetra-O-acetyl-β-D-galactopyranoside

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 10h; | 55.4% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In N-methyl-acetamide; water | 54% |
-
-
4759-48-2
13-cis-retinoic acid

-
-
4753-07-5, 5160-10-1, 14206-57-6, 14227-66-8, 14257-35-3, 42385-75-1, 67650-37-7, 68682-05-3, 69308-57-2, 69349-74-2, 70223-96-0, 70223-97-1, 71276-38-5, 85248-99-3, 118493-15-5, 120575-76-0
2,3,6,2',3',4',6'-hepta-O-acetyl-α-D-maltosyl bromide

| Conditions | Yield |
|---|---|
| With dmap; potassium hydroxide In dichloromethane for 9h; Heating; | 53.1% |

-
-
4759-48-2
13-cis-retinoic acid

| Conditions | Yield |
|---|---|
| 35% |
-
-
4759-48-2
13-cis-retinoic acid

-
-
14857-77-3
L-homocysteic acid

| Conditions | Yield |
|---|---|
| 25% |
Isotretinoin Chemical Properties
IUPAC Name: (2Z,4E,6E,8E)-3,7-Dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl)nona-2,4,6,8-tetraenoic acid
Synonyms of 13-cis-Vitamin A acid (CAS NO.4759-48-2): 13-cis-Vitamin A acid;3,7-Dimethyl-9-(2,6,6-trimethyl-1-cyclohexen-1-yl)2-cis-4-trans-6-trans-8-trans-nonatetraenoic acid ; Accutane ; Isotretinoin ; Isotretinoinum ; Neovitamin A acid
CAS NO: 4759-48-2
Molecular Formula: C20H28O2
Molecular Weight: 300.44
Molecular Structure: 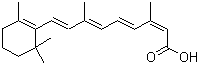
EINECS: 225-296-0
Melting Point: 172-175 °C(lit.)
Storage temp: -20°C
Index of Refraction: 1.556
Molar Refractivity: 95.52 cm3
Molar Volume: 297.1 cm3
Surface Tension: 39.1 dyne/cm
Density: 1.011 g/cm3
Flash Point: 350.6°C
Boiling Point: 462.8 °C at 760 mmHg
Enthalpy of Vaporization: 79.32 kJ/mol
Water Solubility: insoluble
Appearance: Yellow-Orange Crystalline Powder
Stability: Stable, but probably air and light sensitive. Combustible. Incompatible with strong oxidizing agents.
Product Categories of 13-cis-Vitamin A acid (CAS NO.4759-48-2): Antibiotics;Organic acids;APIs;Intermediates & Fine Chemicals;Pharmaceuticals;Retinoids
SMILES: O=C(O)C=C(C=CC=C(C=C/C1=C(/CCCC1(C)C)C)C)C
InChI: InChI=1/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)
InChIKey: SHGAZHPCJJPHSC-UHFFFAOYAP
Std. InChI: InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)
Std. InChIKey: SHGAZHPCJJPHSC-UHFFFAOYSA-N
Isotretinoin Uses
13-cis-Vitamin A acid (CAS NO.4759-48-2) is used as a treatment for severe acne. Presently being studied in conjuction with the treatment of photoaged skin. It is a topical keratolytic agent which is used in the treatment skin diseases including acne vulgaris. It inhibits the secretion of sebum and alters the lipid composition of the skin surface. Besides, it also shows antiinflammatory and antitumor activities by gene regulation.
Isotretinoin Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| child | TDLo | oral | 30mg/kg/21W (30mg/kg) | MUSCULOSKELETAL: OTHER CHANGES SKIN AND APPENDAGES (SKIN): "DERMATITIS, IRRITATIVE: AFTER SYSTEMIC EXPOSURE" SKIN AND APPENDAGES (SKIN): NAILS: OTHER | American Journal of Diseases of Children. Vol. 142, Pg. 316, 1988. |
| child | TDLo | oral | 360mg/kg/26W- (360mg/kg) | SKIN AND APPENDAGES (SKIN): SWEATING: OTHER | CUTIS; Cutaneous Medicine for the Practitioner. Vol. 38, Pg. 275, 1986. |
| man | TDLo | oral | 24mg/kg/4W-I (24mg/kg) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" GASTROINTESTINAL: OTHER CHANGES | Gastroenterology. Vol. 93, Pg. 606, 1987. |
| man | TDLo | oral | 37mg/kg/5W-I (37mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, IRRITATIVE: AFTER SYSTEMIC EXPOSURE" IMMUNOLOGICAL INCLUDING ALLERGIC: DECREASED IMMUNE RESPONSE | Archives of Dermatology. Vol. 122, Pg. 815, 1986. |
| man | TDLo | unreported | 21mg/kg/3W-I (21mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | British Medical Journal. Vol. 290, Pg. 820, 1985. |
| mouse | LD50 | intraperitoneal | 138mg/kg (138mg/kg) | "The Retinoids, Vol.2," Sporn, M.B., et al., eds., New York, Academic Press, Inc., 1984Vol. 2, Pg. 287, 1984. | |
| mouse | LD50 | oral | 3389mg/kg (3389mg/kg) | "The Retinoids, Vol.2," Sporn, M.B., et al., eds., New York, Academic Press, Inc., 1984Vol. 2, Pg. 287, 1984. | |
| rabbit | LD50 | oral | 1960mg/kg (1960mg/kg) | "The Retinoids, Vol.2," Sporn, M.B., et al., eds., New York, Academic Press, Inc., 1984Vol. 2, Pg. 287, 1984. | |
| rat | LD50 | intraperitoneal | 901mg/kg (901mg/kg) | Journal of the American Academy of Dermatology. Vol. 6, Pg. 652, 1982. | |
| rat | LD50 | oral | > 4gm/kg (4000mg/kg) | Journal of the American Academy of Dermatology. Vol. 6, Pg. 652, 1982. | |
| women | TDLo | oral | 56mg/kg/8W-I (56mg/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, IRRITATIVE: AFTER SYSTEMIC EXPOSURE" | CUTIS; Cutaneous Medicine for the Practitioner. Vol. 37, Pg. 115, 1986. |
Isotretinoin Consensus Reports
Reported in EPA TSCA Inventory.
Isotretinoin Safety Profile
Hazard Codes:  T
T
Risk Codes: 61-36/37/38-20/21/22
R61:May cause harm to the unborn child.
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed.
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Codes: 53-26-36/37/39-45
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical
advice.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the
label whenever possible.)
S53: Avoid exposure - obtain special instructions before use.
WGK Germany: 3
RTECS: VH6440000
Poison by intraperitoneal route. Moderately toxic by ingestion. A human teratogen by ingestion with fetal developmental abnormalities of the skin and appendages and other postnatal effects. Human reproductive effects. Human systemic effects: decreased immune response, diarrhea, hypermotility, irritative dermatitis, sweating. Human mutation data reported. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes.
Isotretinoin Specification
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or chemical foam.
Handling: Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
Storage: Do not store in direct sunlight. Store in a tightly closed container. Store in a dry area. Store in freezer.
Related Products
- Isotretinoin
- 475977-79-8
- 475978-44-0
- 475992-33-7
- 475994-60-6
- 476004-79-2
- 476004-81-6
- 476014-76-3
- 476014-92-3
- 4760-34-3
- 4760-35-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View