-
Name
(1R)-(-)-10-Camphorsulfonic acid
- EINECS 252-817-9
- CAS No. 35963-20-3
- Article Data17
- CAS DataBase
- Density 1.331 g/cm3
- Solubility soluble in water
- Melting Point 198 °C (dec.)(lit.)
- Formula C10H16O4S
- Boiling Point 467.7°C at 760 mmHg
- Molecular Weight 232.301
- Flash Point 236.6°C
- Transport Information
- Appearance white to light yellow crystal powder
- Safety 26-36/37/39-45-24/25-27
- Risk Codes 34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms Bicyclo[2.2.1]heptane-1-methanesulfonicacid, 7,7-dimethyl-2-oxo-, (1R)-;(-)-(1R)-7,7-Dimethyl-2-oxobicyclo[2.2.1]heptane-1-methanesulfonic acid;(-)-10-Camphorsulfonic acid;(-)-Camphorsulfonic acid;(1R)-10-Camphorsulfonic acid;(R)-(-)-CSA;(R)-Camphor-10-sulfonic acid;l-10-Camphorsulfonic acid;L-(-) Camphor-10-Sulfonic Acid;
- PSA 79.82000
- LogP 2.35040
Synthetic route
-
-
188416-34-4, 137234-71-0
(2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol (1R)-10-camphorsulfonate

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: (2R,3S)-2-(2,4-difluorophenyl)-3-(5-fluoro-4-pyrimidinyl)-1-(1H-1,2,4-triazol-1-yl)-2-butanol (1R)-10-camphorsulfonate With potassium carbonate In dichloromethane; water pH=8.9; Stage #2: In dichloromethane; cyclohexane at 20 - 50℃; for 8h; Reagent/catalyst; | 93.2% |
-
-
357-57-3
brucine

-
-
5872-08-2
10-camphorsufonic acid

-
A
-
3144-16-9
(1S)-10-camphorsulfonic acid

-
B
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
5872-08-2
10-camphorsufonic acid

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; d-phenylglycine | |
| With brucine |
-
-
76-26-6, 3144-16-9, 5872-08-2, 35963-20-3
camphor-10-sulfonic acid

-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| man zerlegt das in der Mutterlauge vom Brucinsalz der d-Saeure vorgekommene Salz mit Barytwasser; |

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; acetic anhydride |
-
-
7647-01-0
hydrogenchloride

-
-
2935-35-5
(S)-2-phenylglycine

-
-
5872-08-2
10-camphorsufonic acid

-
A
-
3144-16-9
(1S)-10-camphorsulfonic acid

-
B
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
82509-30-6
d-camphor β-sulphonate ammonium salt

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| In water; 4-methyl-2-pentanone at 80 - 117℃; Inert atmosphere; |
-
-
5872-08-2
10-camphorsufonic acid

-
A
-
3144-16-9
(1S)-10-camphorsulfonic acid

-
B
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| With barium(II) perchlorate In methanol Solvent; Reagent/catalyst; Resolution of racemate; |
-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
59543-81-6
4-(1H-imidazol-1-yl)-2-butanone

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 2h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 85℃; for 4h; | 100% |
-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
121352-80-5, 121422-48-8
(2S*,3R*,3'R*,4'R*)-2-(3',4'-epoxy-5'-hexenyl)tetrahydropyran-3-ol

-
-
121352-81-6
((1R,4S)-7,7-Dimethyl-2-oxo-bicyclo[2.2.1]hept-1-yl)-methanesulfonic acid (1R,2R)-2-hydroxy-4-((2S,3R)-3-hydroxy-tetrahydro-pyran-2-yl)-1-vinyl-butyl ester

| Conditions | Yield |
|---|---|
| In dichloromethane for 0.5h; Ambient temperature; | 98% |
-
-
855473-48-2
(R,E)-methyl 2-(3-(acetylthio)-3-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)propyl)benzoate

-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
676-58-4
methylmagnesium chloride

| Conditions | Yield |
|---|---|
| Stage #1: methylmagnesium chloride With cerium(III) chloride In tetrahydrofuran at -20 - 25℃; for 1.75h; Stage #2: (R,E)-methyl 2-(3-(acetylthio)-3-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)propyl)benzoate In tetrahydrofuran; toluene at -20℃; for 0.5h; Stage #3: (R)-10-camphorsulfonic acid | 97.65% |

-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
20667-12-3
silver(l) oxide

-
-
99147-14-5
silver (1R)-(-)-camphor-10-sulfonate

| Conditions | Yield |
|---|---|
| In water an aq. soln. of the camphorsulfonic acid was treated with silver oxide; stirring for 5 min;; centrifugation and decantation; the supernatant was evapd. to dryness; the residue was washed with cold H2O and dried under vac.;; | 96.7% |
-
-
3240-34-4
[bis(acetoxy)iodo]benzene

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| for 0.00555556h; Microwave irradiation; neat (no solvent); | 94% |
| for 0.166667h; | 91% |
-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
41018-45-5
naphthalen-1-yl-λ3-iodanediyl diacetate

| Conditions | Yield |
|---|---|
| for 0.166667h; | 94% |
-
-
639489-84-2
TC-5619

-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
1111941-97-9
(2S,3R)-N-(2-((3-pyridinyl)methyl)-1-azabicyclo[2.2.2]oct-3-yl)benzofuran-2-carboxamide R-camphorsulfonate

| Conditions | Yield |
|---|---|
| In ethanol Heating / reflux; | 93.8% |
-
-
931-53-3
Cyclohexyl isocyanide

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| With water In dichloromethane at 20℃; for 0.333333h; | 93% |
-
-
225382-63-8
[2-(3-Methyl-3H-imidazol-4-yl)-thieno[3,2-b]pyridin-7-yl]-(2-methyl-1H-indol-5-yl)-amine

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; methanol; dichloromethane Large scale; | 93% |
-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| With water In dichloromethane at 20℃; for 0.333333h; | 92% |
-
-
82789-35-3
methyl-1-(1,3-benzodioxol-5-yl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate

-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
1375000-23-9
(1R,3R)-1-(3,4-methylenedioxyphenyl)-2,3,4,9-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylic methyl ester (1R)-10-camphorsulfonic acid salt

| Conditions | Yield |
|---|---|
| In nitromethane at 0℃; Reflux; | 92% |
-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| In acetone at 40℃; for 0.0833333h; Schlenk technique; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| In methanol; isopropyl alcohol at 0℃; for 1.5h; | 92% |
-
-
760937-92-6
((2S,4S)-4-(4-(3-methyl-1-phenyl-1H-pyrazol-5-yl)piperazin-1-yl)pyrrolidin-2-yl)(1,3-thiazolidin-3-yl)methanone

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; tert-butyl methyl ether at 20℃; for 1h; | 91% |
-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
72597-34-3
(+/-)-10-Camphorsulfonamide

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In dichloromethane at 0℃; for 2h; | 90% |
| Stage #1: (R)-10-camphorsulfonic acid With thionyl chloride In chloroform for 17h; Heating / reflux; Stage #2: With ammonia In chloroform; water at 4 - 20℃; for 4h; | 83% |
| Multi-step reaction with 2 steps 1: 80.5 percent / PCl5 / 1 h at 0 deg C and 2 h at r.t. 2: 74 percent / NH3 / toluene / 0 °C View Scheme |
-
-
120-57-0
piperonal

-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
7303-49-3
DL-tryptophan methyl ester

-
-
1375000-23-9
(1R,3R)-1-(3,4-methylenedioxyphenyl)-2,3,4,9-tetrahydro-9H-pyrido[3,4-b]indole-3-carboxylic methyl ester (1R)-10-camphorsulfonic acid salt

| Conditions | Yield |
|---|---|
| In nitromethane at 0 - 30℃; Reflux; | 90% |

-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
75276-67-4
1-(2-methyl-3-chlorophenyl)-4,6-dimethylpyrimidin-2(1H)-thione

-
-
75276-78-7
1-(2-methyl-3-chlorophenyl)-4,6-dimethyl-2-thioxo-1,2-dihydropyrimidinium D-camphor-10-sulphonate

| Conditions | Yield |
|---|---|
| In ethanol for 1h; Heating; | 89% |
-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
83391-98-4, 83435-51-2, 106036-93-5
1-β-dimethylaminoethyl-3,3-bis(trifluoromethyl)diaziridine

-
-
83429-30-5
[2-(3,3-Bis-trifluoromethyl-diaziridin-1-yl)-ethyl]-dimethyl-amine; compound with ((1R,4S)-7,7-dimethyl-2-oxo-bicyclo[2.2.1]hept-1-yl)-methanesulfonic acid

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 0℃; for 24h; | 88.1% |
-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
113665-84-2
(S)-(+)-clopidogrel

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 12h; | 88% |
| In acetone at 20℃; for 4h; | 86% |
| In acetone at 25 - 56℃; for 2.5h; | 85.25% |
| In ethyl acetate at 20℃; for 2h; Product distribution / selectivity; | |
| In acetone at 20℃; for 2h; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| In water; acetonitrile at 60℃; for 4.5h; Solvent; Inert atmosphere; Large scale; | 88% |
-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
28899-97-0
triphenylbismuth(V) diacetate

| Conditions | Yield |
|---|---|
| In acetonitrile addn. of soln. of (1R)-(-)-camphor-10-sulfonic acid (2 equiv.) in MeCN to soln. of Bi(OAc)2Ph3 (1 equiv.) in MeCN; reflux with stirring for 1 h; filtration, removal of solvent under reduced pressure, recrystn. from CH2Cl2-Et2O-pentane; elem. anal.; | 86% |
-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| With sodium azide; trichloroacetonitrile; triphenylphosphine In acetonitrile at 20℃; for 2h; | 86% |

| Conditions | Yield |
|---|---|
| Stage #1: (R)-butane-1,3-diol With trifluoromethylsulfonic anhydride; N-ethyl-N,N-diisopropylamine In acetonitrile at -35 - -30℃; for 3.83333h; Inert atmosphere; Stage #2: Benzhydrylamine In acetonitrile at -35 - 45℃; for 2.66667h; Stage #3: (R)-10-camphorsulfonic acid In methanol at 10 - 20℃; for 0.25h; | 86% |
| Stage #1: (R)-butane-1,3-diol With trifluoromethylsulfonic anhydride; N-ethyl-N,N-diisopropylamine In acetonitrile at -35 - -30℃; for 0.833333h; Stage #2: (R)-10-camphorsulfonic acid; Benzhydrylamine In acetonitrile at -30 - 45℃; | 65% |

-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
112101-81-2
5-((R)-2-amino-1-propyl)-2-methoxybenzenesulphonamide

-
-
906338-31-6
C10H16N2O3S*C10H16O4S

| Conditions | Yield |
|---|---|
| In water; isopropyl alcohol at 0 - 4℃; for 1.5h; Product distribution / selectivity; Heating / reflux; | 85.65% |

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| In methanol at 20 - 50℃; for 2h; Product distribution / selectivity; Heating / reflux; | 85% |
| In methanol; ethyl acetate at 20 - 50℃; for 2h; Product distribution / selectivity; Heating / reflux; | |
| In methanol; isopropyl alcohol at 20 - 50℃; for 2h; Product distribution / selectivity; Heating / reflux; |
-
-
1445-45-0
Trimethyl orthoacetate

-
-
35963-20-3
(R)-10-camphorsulfonic acid

-
-
83603-04-7
methyl 1R-(-)-10-camphorsulphonate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 85% |
| at 20℃; | 85% |
-
-
391-77-5
4-Chloro-6-fluoroquinoline

-
-
17159-79-4
ethyl 4-oxocyclohexane-1-carboxylate

-
-
35963-20-3
(R)-10-camphorsulfonic acid

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 4-oxocyclohexane-1-carboxylate; (R)-10-camphorsulfonic acid In ethylene glycol; toluene at 60℃; for 4h; Inert atmosphere; Dean-Stark; Large scale; Stage #2: 4-Chloro-6-fluoroquinoline With sodium hexamethyldisilazane In tetrahydrofuran; N,N-dimethyl-formamide; toluene at -20℃; for 3h; Large scale; Stage #3: In ethylene glycol Temperature; Large scale; | 84.8% |


| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane at 20℃; for 53h; Product distribution / selectivity; | 84.7% |
| In ethyl acetate at 20℃; for 53h; Product distribution / selectivity; | 83.8% |
| In isopropyl alcohol at 20 - 35℃; for 53h; Product distribution / selectivity; | 82.9% |
L(-)-Camphorsulfonic acid Chemical Properties
(-)-10-CAMPHORSULFONIC ACID;(-)-CAMPHOR-10-SULPHONIC ACID ;(-)-10-CAMPHORSULFONIC ACID;(-)-CAMPHOR-10-SULPHONIC ACID ;
Molecular formula: C10H16O4S
Molecular Weight: 232.3
Molecular Structure :
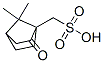
EINECS: 252-817-9
Melting point: 198 °C (dec.)(lit.)
alpha: -22 o (c=20,H2O)
refractive index : -21.5 ° (C=5, H2O)
storage temp.:2-8°C
Water Solubility:soluble
Sensitive:Hygroscopic
L(-)-Camphorsulfonic acid Safety Profile
 C
C The Risk Statements information of L(-)-Camphorsulfonic acid:
34: Causes burns
The Safety Statements information of L(-)-Camphorsulfonic acid:
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
27: Take off immediately all contaminated clothing
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
24/25: Avoid contact with skin and eyes
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
RIDADR:UN 3261 8/PG 2
WGK Germany:3
F:3-10
HazardClass:8
Related Products
- L(-)-Camphorsulfonic acid
- 35963-83-8
- 35964-48-8
- 35966-16-6
- 35966-84-8
- 35967-24-9
- 35967-49-8
- 35969-51-8
- 35969-54-1
- 35970-34-4
- 359-70-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View