-
Name
L-Prolinamide
- EINECS 231-397-0
- CAS No. 7531-52-4
- Article Data43
- CAS DataBase
- Density 1.106 g/cm3
- Solubility Soluble in water and ethanol (50 mg/ml).
- Melting Point 97-102 °C
- Formula C5H10N2O
- Boiling Point 303.6 °C at 760 mmHg
- Molecular Weight 114.147
- Flash Point 137.4 °C
- Transport Information
- Appearance white crystalline powder
- Safety 22-24/25-36/37/39-26
- Risk Codes 22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Prolinamide;(2S)-pyrrolidine-2-carboxamide;H-Pro-NH2;(2S)-2,3,4,5-tetrahydropyrrole-2-carboxamide;(S)-Prolinamide;Pro-NH2;Pro- NH2;L-Prolinmide;
- PSA 55.12000
- LogP 0.25280
Synthetic route
-
-
34079-31-7
Z-Pro-NH2

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With 10% palladium on carbon; hydrogen In methanol for 7h; | 100% |
| With hydrogen; palladium on activated charcoal In methanol for 9h; Ambient temperature; | 88.4% |
| With hydrogen; palladium on activated charcoal In methanol under 2068.6 Torr; for 5h; | 87% |
| With hydrogen; palladium In methanol for 3h; |
-
-
16395-58-7
N-acetyl-L-prolinamide

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 103℃; for 2h; Temperature; | 95.3% |

| Conditions | Yield |
|---|---|
| With ammonia In methanol | 91% |
| With ammonia In methanol at 0 - 20℃; for 15h; Reagent/catalyst; Large scale; | 85% |
| With ammonia In methanol at -25℃; for 96h; Sealed tube; | 55% |

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 65℃; for 15h; | 90% |
| With methanol; ammonia | |
| With ammonium hydroxide | |
| With ammonium hydroxide In butan-1-ol at 5 - 30℃; |
-
-
929897-95-0
(2S,1'S)-1-(1'-phenylethyl)pyrrolidine-2-carboxamide

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium dihydroxide In ethanol; ethyl acetate at 20℃; under 760 Torr; for 14h; | 77% |
-
-
115134-39-9
(S)-(9H-fluoren-9-yl)methyl 2-carbamoylpyrrolidine-1-carboxylate

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With morpholine In N,N-dimethyl-formamide for 1h; | 73% |
| With alkylamine In N,N-dimethyl-formamide at 20℃; for 1h; |

| Conditions | Yield |
|---|---|
| Stage #1: L-proline With thionyl chloride In methanol for 2h; Heating; Stage #2: With ammonia In methanol at 20℃; for 96h; Further stages.; | 72% |
| Multi-step reaction with 3 steps 1: sodium hydroxide / H2O / Ambient temperature 2: 1.) triethylamine, isobutyl chloroformate, 2.) ammonia / 1.) chloroform, 0 deg C, 2 h, 2.) chloroform, RT, overnight 3: 88.4 percent / hydrogen / 10percent palladium on carbon / methanol / 9 h / Ambient temperature View Scheme | |
| Multi-step reaction with 2 steps 1: thionyl chloride / -5 - 70 °C 2: ammonium hydroxide / butan-1-ol / 5 - 30 °C View Scheme |

| Conditions | Yield |
|---|---|
| With ammonium acetate anodic oxidation; Pt electrodes, i = 370 mA*cm-2, current equiv.: 6.2 F/mol; | A 49% B 41% |

| Conditions | Yield |
|---|---|
| With methanol; ammonia |
-
-
125219-05-8
(S)-2-Carbamoyl-pyrrolidine-1-carboxylic acid 2-(4-methoxy-phenyl)-2-oxo-ethyl ester

-
A
-
100-06-1
1-(4-methoxyphenyl)ethanone

-
B
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| Irradiation; mild conditions; |
-
-
35150-07-3
(S)-tert-butyl 2-carbamoylpyrrolidine-1-carboxylate

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 0℃; for 1h; | |
| With trifluoroacetic acid acidolysis; | |
| With trifluoroacetic acid In dichloromethane at 20℃; for 1h; | |
| With trifluoroacetic acid In dichloromethane at 0 - 20℃; for 0.5h; Inert atmosphere; | |
| With trifluoroacetic acid In dichloromethane at 20℃; for 2h; |

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With methanol; ammonia |
-
-
71989-31-6
Fmoc-Pro-OH

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| Stage #1: Fmoc-Pro-OH With Rink amide resin; benzotriazol-1-ol; O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0 - 20℃; for 3.5h; Stage #2: With piperidine In N,N-dimethyl-formamide at 20℃; for 0.416667h; Stage #3: With trifluoroacetic acid | |
| Multi-step reaction with 2 steps 1: pyridine; di-tert-butyl dicarbonate; ammonium bicarbonate / 20 °C 2: alkylamine / N,N-dimethyl-formamide / 1 h / 20 °C View Scheme |
-
-
929897-92-7
(2S,1'S)-5-hydroxy-2-(1'-phenylethylamino)pentanenitrile

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: NEt3; MsCl; DMAP / CH2Cl2 / 36 h / 0 - 20 °C 1.2: 3380 mg / aq. H2SO4 / CH2Cl2 / 48 h / 20 °C 2.1: 77 percent / H2 / Pd(OH)2/C / ethanol; ethyl acetate / 14 h / 20 °C / 760 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: 78 percent / aq. H2SO4 / CH2Cl2 / 48 h / 20 °C 2: 77 percent / H2 / Pd(OH)2/C / ethanol; ethyl acetate / 14 h / 20 °C / 760 Torr View Scheme |
-
-
15761-39-4
1-(tert-butoxycarbonyl)-L-proline

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 99 percent / Boc2O; pyridine; (NH4)2CO3 / dioxane / 18 h / 20 °C 2: TFA / CH2Cl2 / 1 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 88 percent / NH4HCO3; EEDQ / acetonitrile / 15 h / 20 °C 2: 73 percent / morpholine / dimethylformamide / 1 h View Scheme |

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NH3(gas) / CH2Cl2 / 1 h / -20 °C 2: 87 percent / H2 / 5percent Pd/C / methanol / 5 h / 2068.6 Torr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: N-methylmorpholine / CH2Cl2 / 2 h / -20 - -15 °C 2: NH3(gas) / CH2Cl2 / 1 h / -20 °C 3: 87 percent / H2 / 5percent Pd/C / methanol / 5 h / 2068.6 Torr View Scheme | |
| Multi-step reaction with 2 steps 1: 1.) triethylamine, isobutyl chloroformate, 2.) ammonia / 1.) chloroform, 0 deg C, 2 h, 2.) chloroform, RT, overnight 2: 88.4 percent / hydrogen / 10percent palladium on carbon / methanol / 9 h / Ambient temperature View Scheme |
-
-
7732-18-5
water

-
-
17460-56-9
N-[[(Phenylmethoxy)-carbonyl]-D-phenylalanyl]-L-proline

-
-
543-27-1
isobutyl chloroformate

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine In tetrahydrofuran; ethyl acetate | 1.5 g (72%) |

-
-
175446-63-6
proline acid chloride

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 20℃; for 72h; |
-
-
97482-27-4
(5S)-2,2-dimethyl-1,3-diazabicyclo<3.3.0>octan-4-one

-
A
-
174089-62-4
(2S)-N-propan-2-ylpyrrolidine-2-carboxamide

-
B
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol |

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In methanol |

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In chloroform-d1 Inert atmosphere; solid phase reaction; |
-
-
15761-39-4
1-(tert-butoxycarbonyl)-L-proline

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 4-methyl-morpholine; isobutyl chloroformate / tetrahydrofuran / 0.58 h / 0 °C 1.2: 4 h / 0 - 20 °C 2.1: trifluoroacetic acid / dichloromethane / 2 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: thionyl chloride / dichloromethane / 6 h / 3 - 30 °C 2: ammonium hydroxide / dichloromethane / 3 h / 30 °C 3: hydrogenchloride / water / 2 h / 103 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sulfuric acid / 2 h / 1 - 25 °C 2.1: potassium borohydride / water / 4 h / 3 - 45 °C 3.1: chloroformic acid ethyl ester; triethylamine / dichloromethane / 1.5 h / -10 °C 3.2: 0.5 h / 25 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium borohydride / water / 4 h / 3 - 45 °C 2.1: chloroformic acid ethyl ester; triethylamine / dichloromethane / 1.5 h / -10 °C 2.2: 0.5 h / 25 °C View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: L-prolinamide; Wang resin-linked N1-tert-butyloxycarbonyl-N2-triflylguanidine In dichloromethane at 20℃; Stage #2: trifluoroacetic acid In dichloromethane Further stages.; | 100% |

-
-
598-21-0
2-Bromoacetyl bromide

-
-
7531-52-4
L-prolinamide

-
-
253309-37-4
(S)-1-(2-bromoacetyl)pyrrolidine-2-carboxamide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane for 2h; cooling; | 100% |
| Stage #1: 2-Bromoacetyl bromide; L-prolinamide In tetrahydrofuran at 0℃; for 0.5h; Stage #2: With potassium carbonate In tetrahydrofuran at 0 - 20℃; for 4h; | 85.8% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
7531-52-4
L-prolinamide

-
-
35150-07-3
(S)-tert-butyl 2-carbamoylpyrrolidine-1-carboxylate

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; water at 20℃; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 6h; | |
| With potassium carbonate In dichloromethane at 10 - 15℃; Inert atmosphere; | |
| With triethylamine In dichloromethane at -15℃; for 12h; Concentration; | |
| With N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate In dichloromethane at 10 - 15℃; for 2h; |
-
-
10193-84-7
2,4-di-tertbutyl-6-chloromethylphenol

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In acetonitrile for 24h; Inert atmosphere; Schlenk technique; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 18h; | 100% |

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| Stage #1: 2-((4-(4,6-bismorpholine-1,3,5-triazin-2-yl)phenyl)amino)-1H-benzo[d]imidazole-6-carboxylic acid With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 0.666667h; Inert atmosphere; Stage #2: L-prolinamide In N,N-dimethyl-formamide at 80℃; for 8h; Inert atmosphere; | 99.7% |
| Stage #1: 2-((4-(4,6-bismorpholine-1,3,5-triazin-2-yl)phenyl)amino)-1H-benzo[d]imidazole-6-carboxylic acid With O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 0.666667h; Inert atmosphere; Stage #2: L-prolinamide In N,N-dimethyl-formamide at 80℃; for 8h; Inert atmosphere; | 79% |

| Conditions | Yield |
|---|---|
| Stage #1: fumaryl dichloride; L-prolinamide With dmap; triethylamine In dichloromethane at 0 - 20℃; for 16h; Stage #2: With trifluoroacetic anhydride In dichloromethane at 0 - 20℃; for 24 - 48h; | 98% |
-
-
5435-64-3
3,5,5-trimethyl hexanal

-
-
7531-52-4
L-prolinamide

-
-
1378479-21-0
(7aS)-3-(2,4,4-trimethylpentyl)hexahydro-1H-pyrrolo[1,2-c]imidazol-1-one

| Conditions | Yield |
|---|---|
| With triethylamine In methanol for 18h; Inert atmosphere; Reflux; | 98% |
-
-
16251-77-7
3-Phenylbutyraldehyde

-
-
7531-52-4
L-prolinamide

-
-
1084907-50-5
(7aS)-3-(2-phenylpropyl)hexahydro-1H-pyrrolo[1,2-c]imidazol-1-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol at 60℃; for 24h; | 97% |
| With potassium carbonate In ethanol at 60℃; for 24h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With O-(3,4-dihydro-4-oxo-azabenzo-1,2,3-triazinyl)C(NMe2)2*PF6; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 96.5% |
| With 2,4,6-trimethyl-pyridine; [(Me2N)(1H-1,2,3-triazolo[4,5-b]pyridinyl-1)CH=]Me2N*PF6 3-O In N,N-dimethyl-formamide | 96.5% |
| With 2,4,6-trimethyl-pyridine; 1-hydroxy-7-aza-benzotriazole; diisopropyl-carbodiimide In dichloromethane Condensation; | 94.8% |
-
-
79-04-9
chloroacetyl chloride

-
-
7531-52-4
L-prolinamide

-
-
214398-99-9
(S)-1-(2-chloroacetyl) pyrrolidine-2-carboxamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 5℃; for 2h; Reagent/catalyst; Inert atmosphere; | 96% |
| With triethylamine In dichloromethane at -5 - 0℃; for 0.5h; Inert atmosphere; | 96.3% |
| In tetrahydrofuran at 0℃; Reflux; Large scale; | 92% |
-
-
7051-34-5
cyclopropylcarbinyl bromide

-
-
7531-52-4
L-prolinamide

-
-
114812-44-1
(-)-(S)-1-cyclopropylmethyl-2-pyrrolidinecarboxamide

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 24h; | 96% |
-
-
4530-20-5
BOC-glycine

-
-
7531-52-4
L-prolinamide

-
-
52293-28-4
(S)-tert-butyl 2-(2-carbamoylpyrrolidine-1-yl)-2-oxoethylcarbamate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; | 95.8% |
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; | 95.8% |
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; | 95.8% |
| With benzotriazol-1-ol; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; | 95.8% |
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane; N,N-dimethyl-formamide at 20℃; for 3h; | 71% |
-
-
5491-18-9, 17609-52-8, 53990-33-3, 60584-76-1
(S)-N-(benzyloxycarbonyl)phenylglycine

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With N-(3-dimethylaminopropyl)-N-ethylcarbodiimide; 3-hydroxy-4-oxo-3,4-dihydro-5-azabenzo-1,2,3-triazine In chloroform; 2,2,2-trifluoroethanol at 20℃; | 95.4% |
| Stage #1: (S)-N-(benzyloxycarbonyl)phenylglycine With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 0℃; for 0.166667h; Stage #2: L-prolinamide In N,N-dimethyl-formamide at 20℃; Further stages.; | 93% |
| With 1-hydroxy-7-aza-benzotriazole; N-ethyl-N,N-diisopropylamine; 1-diethoxyphosphinyloxy-7-azabenzotriazole | 92.8% |

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Reflux; | 95% |

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one at 150℃; for 5h; Microwave irradiation; | 95% |
-
-
88-16-4
1-chloro-2-(trifluoromethyl)benzene

-
-
7531-52-4
L-prolinamide

-
-
403478-68-2
(S)-N'-((2-trifluoromethyl)phenyl)prolinylamide

| Conditions | Yield |
|---|---|
| With 4-diphenylphosphino-13-dicyclohexylphosphino-[2.2]paracyclophane; water; palladium diacetate; caesium carbonate In 1,4-dioxane at 125℃; for 24h; Buchwald-Hartwig coupling; | 94% |
-
-
13123-00-7
(2S)-2-[((2S)-2-{[(benzyloxy)carbonyl]amino}-3-phenylpropanoyl)amino]-3-methylbutanoic acid

-
-
7531-52-4
L-prolinamide

-
-
164861-48-7
Z-L-Phe-L-Val-L-Pro-NH2

| Conditions | Yield |
|---|---|
| With 1-hydroxy-7-aza-benzotriazole; [Me2N(1H-1,2,3-triazolo[4,5-b]pyridinyl)CH=]Me2N 3-oxide*PF6; 4-(N,N-dimethylamino)-2,6-di-tert-butylpyridine In N,N-dimethyl-formamide at 20℃; | 93.5% |
| With 2,4,6-trimethyl-pyridine; 1-hydroxy-7-aza-benzotriazole; 1-diethoxyphosphinyloxy-7-azabenzotriazole In N,N-dimethyl-formamide | 93.4% |
| With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide Product distribution; Further Variations:; Reagents; | 87.4% |
-
-
1416564-42-5
4-oxo-1-pentyl-7-(phenylthio)-1,4-dihydroquinoline-3-carboxylic acid

-
-
7531-52-4
L-prolinamide

-
-
1416564-35-6
C26H29N3O3S

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 18.5h; Inert atmosphere; | 93% |

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 18h; | 93% |

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 18h; | 92.9% |

| Conditions | Yield |
|---|---|
| Stage #1: L-prolinamide With hydrogenchloride for 10h; Heating; Stage #2: With methyloxirane In ethanol at 20℃; for 5h; | 92% |
-
-
79-04-9
chloroacetyl chloride

-
-
7531-52-4
L-prolinamide

-
-
207557-35-5
(S)-1-(2-chloroacetyl)pyrrolidine-2-carbonitrile

| Conditions | Yield |
|---|---|
| Stage #1: chloroacetyl chloride; L-prolinamide With potassium carbonate In acetonitrile at 0 - 15℃; Stage #2: With trifluoroacetic anhydride at 0 - 15℃; | 92% |
| Stage #1: chloroacetyl chloride; L-prolinamide With potassium carbonate In acetonitrile at 0 - 20℃; Stage #2: With trifluoroacetic anhydride at 0 - 25℃; | 92% |
| Stage #1: chloroacetyl chloride; L-prolinamide With trifluoroacetyl chloride In N,N-dimethyl-formamide at 50℃; for 1.5h; Stage #2: With trifluoroacetyl chloride In N,N-dimethyl-formamide at 60℃; for 1.5h; Reagent/catalyst; Temperature; | 83.4% |
-
-
1011521-39-3
(2S)-2-[(t-butoxycarbonyl)amino]-3-{4-[(5-nitropyridin-2-yl)oxy]phenyl}propanoic acid

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| Stage #1: (2S)-2-[(t-butoxycarbonyl)amino]-3-{4-[(5-nitropyridin-2-yl)oxy]phenyl}propanoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: L-prolinamide In N,N-dimethyl-formamide at 20℃; for 15h; | 92% |
-
-
1215841-60-3
9-bromo-10-(bromomethyl)-2,3,6-trimethoxyphenanthrene

-
-
7531-52-4
L-prolinamide

-
-
1309782-29-3
(2S)-1-[(10-bromo-3,6,7-trimethoxy-9-phenanthryl)methyl]pyrrolidine-2-carboxamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 8h; Reflux; | 92% |
-
-
1215841-59-0
9-bromo-10-(bromomethyl)-2,3,6,7-tetramethoxyphenanthrene

-
-
7531-52-4
L-prolinamide

-
-
1309782-28-2
(S)-1-[(9-bromo-2,3,6,7-tetramethoxyphenanthren-10-yl)methyl]pyrrolidine-2-carboxamide

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 8h; Reflux; | 92% |
| With potassium carbonate In N,N-dimethyl-formamide for 8h; Reflux; | 92% |

-
-
7531-52-4
L-prolinamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 18h; | 92% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 18h; | 92% |
L-Prolinamide Chemical Properties
IUPAC Name of L-Prolinamide (CAS NO.7531-52-4): pyrrolidine-2-carboxamide
Molecular Formula: C5H10N2O
Molecular Weight: 114.15 g/mol
Following is the molecular structure of L-Prolinamide (CAS NO.7531-52-4):
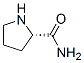
BRN: 80807
EINECS: 231-397-0
Density: 1.106 g/cm3
Flash Point: 137.4 °C
Index of Refraction: 1.491
Melting Point: 95-97 °C(lit.)
Surface Tension: 41.4 dyne/cm
Solubility in water: Very soluble
Enthalpy of Vaporization: 54.39 kJ/mol
Boiling Point: 303.6 °C at 760 mmHg
Vapour Pressure: 0.000923 mmHg at 25 °C
Appearance: white to off-white microcrystalline powder
Product Categories: Aminoacids Derivatives;APIs;Amino Acids;Proline [Pro, P];Amino Acids and Derivatives;Amides (Chiral);Chiral Building Blocks;Synthetic Organic Chemistry;Amino Acid Derivatives;Peptide Synthesis;Proline
L-Prolinamide Toxicity Data With Reference
L-Prolinamide (CAS NO.7531-52-4) hasn't been listed as a carcinogen by ACGIH, IARC, NTP, or CA Prop 65. And the toxicological properties have not been fully investigated.
L-Prolinamide Safety Profile
Safty informations about L-Prolinamide (7531-52-4):
Hazard Codes:  Xn ( Harmful)
Xn ( Harmful)
Risk Statements: 22-36/37/38
R22:Harmful if swallowed
R36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements: 22-24/25
S22:Do not breathe dust
S24/25:Avoid contact with skin and eyes
WGK Germany: 3
L-Prolinamide Specification
L-Prolinamide , its cas register number is 7531-52-4. It also can be called Proline-nh2 ; Prolinamide ; (s)-Pyrrolidine-2-carboxylic acid amide ; (s)-Prolinamide ; H-Pro-nh2 ; L-Proline amide ; L-(-)-Prolinamide;l-prolinamide .
L-Prolinamide (CAS NO.7531-52-4) is stable under normal temperatures and pressures. L-Prolinamide (CAS NO.7531-52-4) should avoid the condition like incompatible materials. It is incompatible with other materials strong oxidizing agents. Its hazardous decomposition products are nitrogen oxides, carbon monoxide, carbon dioxide. Its hazardous polymerization has not been reported.
Related Products
- L-Prolinamide
- L-Prolinamide hydrochloride
- L-Prolinamide, N-((phenylmethoxy)carbonyl)-O-((phenylmethyl)-L-seryl-L-tryptophyl-L-seryl-L-tyrosyl-O-methyl-D-tyrosyl-L-leucyl-L-arginyl-N-ethyl-, monoacetate (salt), dihydrate
- L-Prolinamide, N-((phenylmethoxy)carbonyl)-O-(phenylmethyl)-L-seryl-L-tryptophyl-L-seryl-L-tyrosyl-2,3,4,5,6-pentamethyl-DL-phenylalanyl-L-leucyl-L-arginyl-N-ethyl-, monoacetate (salt),dihydrate
- L-Prolinamide, N-((phenylmethoxy)carbonyl)-O-(phenylmethyl)-L-seryl-L-tryptophyl-L-seryl-L-tyrosyl-2,3,4,5,6-pentamethyl-D-phenylalanyl-L-leucyl-L-arginyl-N-ethyl-, monoacetate (salt),hydrate
- L-Prolinamide, N-((phenylmethoxy)carbonyl)-O-(phenylmethyl)-L-seryl-L-tryptophyl-L-seryl-L-tyrosyl-D-phenylalanyl-L-leucyl-L-arginyl-N,N-dimethyl-, monoacetate (salt), hydrate
- 7531-76-2
- 75321-08-3
- 75321-19-6
- 75323-55-6
- 75330-75-5
- 75-33-2
- 7533-40-6
- 75336-86-6
- 75338-42-0
- 75-34-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View