-
Name
L-Threonine
- EINECS 200-774-1
- CAS No. 72-19-5
- Article Data238
- CAS DataBase
- Density 1.307 g/cm3
- Solubility 90 g/L (20 °C) in water
- Melting Point 256 °C (dec.)(lit.)
- Formula C4H9NO3
- Boiling Point 345.803 °C at 760 mmHg
- Molecular Weight 119.12
- Flash Point 162.936 °C
- Transport Information
- Appearance White crystalline powder
- Safety 24/25-37/39-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Butanoic acid, 2-amino-3-hydroxy-, (R-(R*,S*))-;Thr;Threonine [USAN:INN];L-Threonin;(2S,3R)-2-azaniumyl-3-hydroxy-butanoate;Treonina [Spanish];(2R,3R)-2-amino-3-hydroxy-butanoic acid;Threonine, L-;(2S,3R)-2-Amino-3-hydroxybutyric acid;Valine,3-hydroxy-;L-alpha-Amino-beta-hydroxybutyric acid;(2S,3S)-2-azaniumyl-3-hydroxy-butanoate;(2S)-threonine;Threoninum [Latin];Threonine (VAN);L-2-Amino-3-hydroxybutyric acid;L-Threonine(Feed Grade);L-Thr-OH;L-Threonine Aji97;Threonine, L- (8CI);(2S,3R)-2-amino-3-hydroxy-butanoic acid;L-(-)-Threonine;Threonin;[R-(R*,S*)]-2-Amino-3-hydroxybutanoic acid;L-Threonine (9CI);Threonine (USP);
- PSA 83.55000
- LogP -0.52060
Synthetic route

| Conditions | Yield |
|---|---|
| In pyridine Product distribution; | A 83.8% B n/a |

-
-
67-56-1
methanol

-
-
73731-37-0, 130674-54-3, 146306-75-4
Fmoc-Thr-OH

-
A
-
72-19-5
L-threonine

-
B
-
2812-27-3, 2812-28-4, 21148-57-2, 95539-41-6, 100157-57-1
N-methyl threonine

-
C
-
2812-36-4, 2812-37-5, 138406-48-1
N,N-dimethyl-threonine

| Conditions | Yield |
|---|---|
| With hydrogen; acetic acid; palladium on activated charcoal for 48h; other (protected) amino acids; other alcohol; | A n/a B 40% C n/a |


-
A
-
72-19-5
L-threonine

| Conditions | Yield |
|---|---|
| Yields of byproduct given; |

| Conditions | Yield |
|---|---|
| With pyridoxal 5'-phosphate; L-threonine aldolase In phosphate buffer at 25℃; for 3h; |

-
-
72-19-5
L-threonine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water at 110℃; for 20h; |

-
-
72-19-5
L-threonine

| Conditions | Yield |
|---|---|
| With ferrous(II) sulfate heptahydrate; cobalt(II) sulphate heptahydrate; N-succinyl L-amino acid desuccinylase from Burkholderia ambifaria AMMD; N-succinyll-amino acid β-hydroxylase from Burkholderia ambifaria AMMD; ascorbic acid In aq. phosphate buffer at 30℃; for 24h; Enzymatic reaction; |
-
-
67-56-1
methanol

-
-
72-19-5
L-threonine

-
-
39994-75-7, 60538-15-0, 60538-18-3, 62076-66-8, 76419-75-5, 79617-27-9, 108724-12-5
L-threonine methyl ester hydrochloride

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0℃; for 3.25h; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; sodium carbonate In methanol; dichloromethane | 98% |
| With sodium hydrogencarbonate In diethyl ether; water |

-
-
72-19-5
L-threonine

| Conditions | Yield |
|---|---|
| In methanol byproducts: acetylacetone; to a soln. of complex in methanol was added amino acid, the mixt. was refluxed for 3 h, cooled; evapd. to dryness, treated with diethyl ether, the solid was filtered, washed with diethyl ether, air-dried; | 85% |
-
-
72-19-5
L-threonine

-
-
104-15-4
toluene-4-sulfonic acid

-
-
107-18-6
allyl alcohol

-
-
88224-11-7
L-Threonin-allylester-hydro-p-toluolsulfonat

| Conditions | Yield |
|---|---|
| In benzene Heating; | 84% |

-
-
7647-01-0
hydrogenchloride

-
-
16940-66-2
sodium tetrahydroborate

-
-
12093-10-6
ferrocenecarboxaldehyde

-
-
72-19-5
L-threonine

| Conditions | Yield |
|---|---|
| With LiOH*H2O In methanol L-threonine and LiOH*H2O in dry MeOH stirred for 30 min; MeOH soln. of Fe complex added dropwise; stirred for 20 min; treated with NaBH4 with stirring; solvent evapd.; dissolved in H2O; acidified with dilute HCl (pH 5-7); filtered; ppt. washed with H2O; dried in vac. desiccator; elem. anal.; | 84% |


| Conditions | Yield |
|---|---|
| In pyridine; water for 3h; Ambient temperature; | A n/a B 75% |


| Conditions | Yield |
|---|---|
| In ethanol salicylaldehyde added with stirring to mixt. of Co complex and amino acid in EtOH; refluxed for 3 h; filtered; filtrate concd. in vac.; residue washed with Et2O; dissolved in EtOH; column chromd. (Al2O3, EtOH); purified by gel chromy. (Sephadex LH-20, EtOH-benzene 1:3); elem. anal.; | 70% |


| Conditions | Yield |
|---|---|
| In ethanol aldehyde was added with stirring to mixt. of Co complex and amino acid in EtOH; refluxed for 3 h; filtered; evapd. (vac.); washed (Et2O); dissolved in EtOH; chromd. (Al2O3, EtOH); chromd. (gel, EtOH/C6H6, 1/3); dissolved in aq. EtOH; passed through ion-exchange resin; evapd. (vac.); chromd. (gel, EtOH/C6H6, 1/3);elem. anal.; | 70% |

-
-
1445-73-4
1-Methyl-4-piperidone

-
-
72-19-5
L-threonine

-
-
119072-54-7, 2769-71-3
2,6-dimethylphenyl isonitrile

| Conditions | Yield |
|---|---|
| With triethylamine In 2,2,2-trifluoroethanol at 100℃; for 24h; | 70% |


-
-
72-19-5
L-threonine

-
-
1259524-22-5
3-allyl-5-bromosalicylaldehyde

| Conditions | Yield |
|---|---|
| In ethanol aldehyde was added with stirring to mixt. of Co complex and amino acid in EtOH; refluxed for 3 h; filtered; evapd. (vac.); washed (Et2O); dissolved in EtOH; chromd. (Al2O3, EtOH); chromd. (gel, EtOH/C6H6, 1/3); dissolved in aq. EtOH; passed through ion-exchange resin; evapd. (vac.); chromd. (gel, EtOH/C6H6, 1/3);elem. anal.; | A 22% B 65% |

-
-
72-19-5
L-threonine

-
-
71942-48-8
6-nitro-2-oxo-2H-chromene-3-carbonyl chloride

-
-
80613-36-1
6-nitrocoumarin-3-CO-L-Thr

| Conditions | Yield |
|---|---|
| With triethylamine In 1,4-dioxane 1) room temperature, 2) 2 h, reflux; | 54% |

-
-
72-19-5
L-threonine

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide 120-130°C, 1.0 h; cooling, filtration into ether (sepn. of oil), decantation, washing (ether), extn. into Me2CO, filtration, chromy. (Al2O3, Me2CO, then MeOH/H2O=5:1), evapn. (reduced pressure, 60-70°C); | 40% |

-
-
72-19-5
L-threonine

| Conditions | Yield |
|---|---|
| With NaOH In water stoichiometric equiv. of Ni(ClO4)2*6H2O and L-threonine added to aq. soln. of La(ClO4)3*6H2O; pH adjusted to 6.6 by slow addition of NaOH aq. soln.; stirred for 2 h; filtered to remove precipitates formed; placed in a desiccator filled with P2O5 for 1 month; elem. anal.; | 35% |


-
-
72-19-5
L-threonine

| Conditions | Yield |
|---|---|
| With NaOH In water adjusting the pH of an aq. soln. of the CoBr2 complex and (S)-thr to 8.0 with NaOH, evapn. to near dryness (after 1 h), addn. of a slight excess of an aq. NaClO4 soln., crystn.; elem. anal.; | 30% |

-
-
72-19-5
L-threonine

-
-
501-53-1
benzyl chloroformate

-
-
19728-63-3
N-[(phenylmethoxy)carbonyl]-L-threonine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate |
-
-
492-61-5
β-D-glucose

-
-
72-19-5
L-threonine

-
A
-
320-77-4
1-hydroxy-propane-1,2,3-tricarboxylic acid

-
C
-
77-92-9
citric acid

| Conditions | Yield |
|---|---|
| With Saccharomycopsis lipolytica In water at 26℃; for 120h; Product distribution; also in n-hexadecane medium; catabolism of amino acids by S. lipolytica; |

-
-
66065-85-8
succinimidyl 2,2,2-trichloroethyl carbonate

-
-
72-19-5
L-threonine

-
-
88050-12-8
N-(2,2,2-trichloroethoxycarbonyl)-threonine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium hydrogencarbonate In 1,4-dioxane for 0.166667h; Ambient temperature; |
-
-
72-19-5
L-threonine

-
-
17350-66-2
N-benzyloxycarbonylalaninehydrazid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tert.-butylnitrite; triethylamine 1. DMF, dioxane, -10 degC, 10 min, 2.) H2O, cold; Yield given. Multistep reaction; |
-
-
72-19-5
L-threonine

-
-
86895-16-1
[(R)-1-[(S)-1-(Hydrazinocarbonylmethyl-carbamoyl)-ethylcarbamoyl]-2-(4-methoxy-benzylsulfanyl)-ethyl]-carbamic acid tert-butyl ester

-
-
86905-27-3
Boc-Cys(MBzl)-Ala-Gly-Thr-OH

| Conditions | Yield |
|---|---|
| With hydrogenchloride; tert.-butylnitrite; triethylamine 1.)DMF, dioxane, -25 degC, 10 min, 2.) H2O, cold; Yield given. Multistep reaction; |
-
-
72-19-5
L-threonine

-
-
24830-94-2
D-allo-threonine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanethiol at 110℃; Rate constant; various times; |
-
-
72-19-5
L-threonine

-
-
849704-22-9
(2S,3R)-2-chloro-3-hydroxybutanoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium nitrite at -5℃; for 5h; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; chloroamine-T In water at 29.9 - 44.9℃; Thermodynamic data; Rate constant; Ea, log A, -ΔS(excit.), ΔH(excit.), ΔG(excit.); further acid concentration, further acid and alkaline media; |
-
-
72-19-5
L-threonine

| Conditions | Yield |
|---|---|
| With hydroxide In water Mechanism; Ambient temperature; Irradiation; examination of effect of protonation state of amino group; |

| Conditions | Yield |
|---|---|
| Yield given; |

| Conditions | Yield |
|---|---|
| With thionyl chloride 1)r.t., methanol, 2) 40 deg C 30 min, water; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With hydroxide |
L-Threonine Chemical Properties
Structure of L-Threonine (CAS NO.72-19-5):
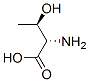
IUPAC Name: (2S,3R)-2-amino-3-hydroxybutanoic acid
Empirical Formula: C4H9NO3
Molecular Weight: 119.1192
EINECS: 200-774-1
Index of Refraction: 1.507
Molar Refractivity: 27.13 cm3
Molar Volume: 91.1 cm3
Polarizability: 10.75×10-24cm3
Surface Tension: 60 dyne/cm
Density: 1.307 g/cm3
Flash Point: 162.9 °C
Enthalpy of Vaporization: 68.32 kJ/mol
Melting Point: 256 °C (dec.)(lit.)
Boiling Point: 345.8 °C at 760 mmHg
Vapour Pressure: 3.77E-06 mmHg at 25°C
Storage temp: Store at RT.
Water Solubility: 90 g/L (20 ºC)
Stability: Stable. Incompatible with strong oxidizing agents.
Physical Appearance: White crystalline powder
Product Categories: Miscellaneous Biochemicals;Amino Acid Derivatives;Threonine [Thr, T];Amino Acids and Derivatives;alpha-Amino Acids;Amino Acids;Biochemistry;Nutritional Supplements;L-Amino Acids;Amino Acids
Synonyms of L-Threonine (CAS NO.72-19-5): (S)-Threonine ; 2-Amino-3-hydroxybutanoic acid, (R-(R*,S*))- ; Butanoic acid, 2-amino-3-hydroxy-, (R-(R*,S*))- ; Threonin ; Threonine ; Threoninum ; Treonina
Canonical SMILES: CC(C(C(=O)O)N)O
Isomeric SMILES: C[C@H]([C@@H](C(=O)O)N)O
InChI: InChI=1S/C4H9NO3/c1-2(6)3(5)4(7)8/h2-3,6H,5H2,1H3,(H,7,8)/t2-,3+/m1/s1
InChIKey: AYFVYJQAPQTCCC-GBXIJSLDSA-N
L-Threonine Uses
L-Threonine (CAS NO.72-19-5) is an essential amino acid, but not synthesized in humans.
L-Threonine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| rat | LD50 | intraperitoneal | 3098mg/kg (3098mg/kg) | BEHAVIORAL: MUSCLE CONTRACTION OR SPASTICITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Archives of Biochemistry and Biophysics. Vol. 58, Pg. 253, 1955. |
L-Threonine Safety Profile
Hazard Codes: Xi
Xi
Risk Statements: 36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 24/25-37/39-26
24/25: Avoid contact with skin and eyes
37/39: Wear suitable gloves and eye/face protection
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
WGK Germany: 3
Hazard Note: Irritant
HS Code: 29225000
L-Threonine Specification
1. First Aid Measures of L-Threonine (CAS NO.72-19-5):
Ingestion: If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation: Remove from exposure to fresh air immediately. Get medical aid if cough or other symptoms appear.
Skin: Get medical aid if irritation develops or persists. Flush skin with plenty of soap and water.
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
2. Handling and Storage:
Storage: Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Handling: Wash thoroughly after handling. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Related Products
- L-Threonine
- L-Threonine
- L-Threonine benzyl ester hemioxalate
- L-Threonine benzyl ester oxalate
- L-Threonine ethyl ester hydrochloride
- L-Threonine hydrochloride
- L-Threonine tert-butyl ester hydrochloride
- L-Threonine, 4-fluoro-
- L-Threonine,L-alanyl-L-glutaminyl-L-a-aspartyl-L-phenylalanyl-L-valyl-L-glutaminyl-L-tryptophyl-L-leucyl-L-methionyl-L-asparaginyl-
- L-Threonine,L-lysyl-D-prolyl-
- 721958-70-9
- 72196-32-8
- 721970-36-1
- 72197-89-8
- 72198-83-5
- 72199-98-5
- 72200-74-9
- 72204-43-4
- 72207-75-1
- 7220-79-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View