-
Name
Loxoprofen sodium
- EINECS 1806241-263-5
- CAS No. 80382-23-6
- Article Data10
- CAS DataBase
- Density
- Solubility
- Melting Point
- Formula C15H17NaO3
- Boiling Point 417.9 °C at 760 mmHg
- Molecular Weight 268.288
- Flash Point 220.7 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Benzeneaceticacid, a-methyl-4-[(2-oxocyclopentyl)methyl]-,sodium salt (9CI);CS 600;CS 600 (antiinflammatory);Loxonin;Loxoprofensodium;Sodium 2-[4-[(2-Oxocyclopentyl)methyl]phenyl]propionate;Sodiumloxoprofen;
- PSA 57.20000
- LogP 1.45170
Synthetic route

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water for 2h; Reflux; | 96.6% |
| With methanol; sodium hydroxide for 1h; | 95% |
| With sodium hydroxide In ethanol at 50℃; for 2h; | 90.3% |
-
-
111128-12-2
2-(4-(bromomethyl)phenyl)propanoic acid

-
-
611-10-9
2-ethoxycarbonyl-1-cyclopentanone

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Stage #1: 2-[4-(bromomethyl)phenyl]propanoic acid; 2-ethoxycarbonyl-1-cyclopentanone With sodium hydroxide In N,N-dimethyl-formamide under 760.051 Torr; for 8h; Stage #2: With hydrogenchloride In water; N,N-dimethyl-formamide pH=5 - 6; Further stages; | 92% |
-
-
2362-36-9
1-(1-chloroethyl)-4-methylbenzene

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: magnesium / tetrahydrofuran / 1.5 h / 20 - 30 °C / Inert atmosphere 2: N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) / dichloromethane / Reflux 3: sulfuric acid / 16 h / 0 - 5 °C 4: sodium periodate / N,N-dimethyl-formamide / 150 °C 5: hydrogenchloride / water / 4.5 h / Reflux 6: 5%-palladium/activated carbon; hydrogen / methanol / 14 h / 10 - 15 °C / 1125.11 Torr / Autoclave 7: sodium hydroxide / ethanol / 1 h / Reflux View Scheme |
-
-
938-94-3
(R,S)-2-(4'-methylphenyl) propionic acid

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) / dichloromethane / Reflux 2: sulfuric acid / 16 h / 0 - 5 °C 3: sodium periodate / N,N-dimethyl-formamide / 150 °C 4: hydrogenchloride / water / 4.5 h / Reflux 5: 5%-palladium/activated carbon; hydrogen / methanol / 14 h / 10 - 15 °C / 1125.11 Torr / Autoclave 6: sodium hydroxide / ethanol / 1 h / Reflux View Scheme |
-
-
111128-12-2
2-(4-(bromomethyl)phenyl)propanoic acid

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: sulfuric acid / 16 h / 0 - 5 °C 2: sodium periodate / N,N-dimethyl-formamide / 150 °C 3: hydrogenchloride / water / 4.5 h / Reflux 4: 5%-palladium/activated carbon; hydrogen / methanol / 14 h / 10 - 15 °C / 1125.11 Torr / Autoclave 5: sodium hydroxide / ethanol / 1 h / Reflux View Scheme | |
| Multi-step reaction with 4 steps 1.1: sulfuric acid / toluene / 35 °C 1.2: 5 h / 281 °C 2.1: sodium methylate / toluene; dimethyl sulfoxide; methanol / 1.5 h / 2 - 5 °C 2.2: 5 - 18 °C 3.1: acetic acid; sulfuric acid / water / 2 h / 110 °C 4.1: sodium hydroxide / acetone / 30 °C / pH 7.2 View Scheme | |
| Multi-step reaction with 4 steps 1: sulfuric acid / 6 h / 0 - 5 °C 2: potassium carbonate / toluene / 12 h / Reflux 3: hydrogen bromide; acetic acid / 8 h / Reflux 4: sodium hydroxide / ethanol; water / 3 h / 20 °C / pH 7 - 8 View Scheme | |
| Multi-step reaction with 4 steps 1: sulfuric acid / 6 h / Cooling with ice 2: toluene / 12 h / 110 °C 3: sodium hydroxide; water / toluene / 2 h / 80 °C 4: sodium hydroxide / ethanol / 2 h / 50 °C View Scheme |
-
-
99807-54-2
2-(4-bromomethylphenyl)propionic acid methyl ester

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: sodium periodate / N,N-dimethyl-formamide / 150 °C 2: hydrogenchloride / water / 4.5 h / Reflux 3: 5%-palladium/activated carbon; hydrogen / methanol / 14 h / 10 - 15 °C / 1125.11 Torr / Autoclave 4: sodium hydroxide / ethanol / 1 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: sodium methylate / toluene; dimethyl sulfoxide; methanol / 1.5 h / 2 - 5 °C 1.2: 5 - 18 °C 2.1: acetic acid; sulfuric acid / water / 2 h / 110 °C 3.1: sodium hydroxide / acetone / 30 °C / pH 7.2 View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium carbonate / acetone 2.1: hydrogenchloride / acetic acid / Reflux 2.2: Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: potassium carbonate / toluene / 12 h / Reflux 2: hydrogen bromide; acetic acid / 8 h / Reflux 3: sodium hydroxide / ethanol; water / 3 h / 20 °C / pH 7 - 8 View Scheme | |
| Multi-step reaction with 3 steps 1: toluene / 12 h / 110 °C 2: sodium hydroxide; water / toluene / 2 h / 80 °C 3: sodium hydroxide / ethanol / 2 h / 50 °C View Scheme |
-
-
63476-54-0
2-(4-formylphenyl)propionic acid methyl ester

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrogenchloride / water / 4.5 h / Reflux 2: 5%-palladium/activated carbon; hydrogen / methanol / 14 h / 10 - 15 °C / 1125.11 Torr / Autoclave 3: sodium hydroxide / ethanol / 1 h / Reflux View Scheme |
-
-
81762-92-7
loxoprofen methyl ester

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol for 1h; Reflux; | 35.36 g |

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: magnesium; iodine / tetrahydrofuran / 20 - 25 °C 1.2: 0 - 20 °C 2.1: hydrogenchloride / methanol; water / 0 °C / Reflux 2.2: 0 °C / Reflux 3.1: sodium hydroxide; methanol / 1 h View Scheme |
-
-
589-15-1
1-bromomethyl-4-bromobenzene

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: sodium methylate / methanol / Reflux 2.1: acetic acid; hydrogenchloride / water / Reflux 3.1: pyridinium p-toluenesulfonate / methanol / 45 - 50 °C 4.1: magnesium; iodine / tetrahydrofuran / 20 - 25 °C 4.2: 0 - 20 °C 5.1: hydrogenchloride / methanol; water / 0 °C / Reflux 5.2: 0 °C / Reflux 6.1: sodium hydroxide; methanol / 1 h View Scheme |
-
-
10472-24-9
methyl 2-oxocyclopentane-1-carboxylate

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: sodium methylate / methanol / Reflux 2.1: acetic acid; hydrogenchloride / water / Reflux 3.1: pyridinium p-toluenesulfonate / methanol / 45 - 50 °C 4.1: magnesium; iodine / tetrahydrofuran / 20 - 25 °C 4.2: 0 - 20 °C 5.1: hydrogenchloride / methanol; water / 0 °C / Reflux 5.2: 0 °C / Reflux 6.1: sodium hydroxide; methanol / 1 h View Scheme |

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: acetic acid; hydrogenchloride / water / Reflux 2.1: pyridinium p-toluenesulfonate / methanol / 45 - 50 °C 3.1: magnesium; iodine / tetrahydrofuran / 20 - 25 °C 3.2: 0 - 20 °C 4.1: hydrogenchloride / methanol; water / 0 °C / Reflux 4.2: 0 °C / Reflux 5.1: sodium hydroxide; methanol / 1 h View Scheme |
-
-
500764-03-4
2-(4-bromobenzyl)cyclopentanone

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: pyridinium p-toluenesulfonate / methanol / 45 - 50 °C 2.1: magnesium; iodine / tetrahydrofuran / 20 - 25 °C 2.2: 0 - 20 °C 3.1: hydrogenchloride / methanol; water / 0 °C / Reflux 3.2: 0 °C / Reflux 4.1: sodium hydroxide; methanol / 1 h View Scheme |
-
-
79443-97-3
methyl 2-(4-methylphenyl)propanoate

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: N-Bromosuccinimide; 2,2'-azobis(isobutyronitrile) / tetrachloromethane / Reflux 2.1: sodium carbonate / acetone 3.1: hydrogenchloride / acetic acid / Reflux 3.2: Reflux View Scheme |

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Stage #1: C18H22O5 With hydrogenchloride In acetic acid Reflux; Stage #2: With sodium hydroxide In ethanol Reflux; |
-
-
120-92-3
cyclopentanone

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: toluene-4-sulfonic acid / toluene / 8 h / 110 °C 2: toluene / 12 h / 110 °C 3: sodium hydroxide; water / toluene / 2 h / 80 °C 4: sodium hydroxide / ethanol / 2 h / 50 °C View Scheme |
-
-
936-52-7
1-(N-morpholino)cyclopent-1-ene

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: toluene / 12 h / 110 °C 2: sodium hydroxide; water / toluene / 2 h / 80 °C 3: sodium hydroxide / ethanol / 2 h / 50 °C View Scheme |
-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Stage #1: sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate With pyrographite In ethanol; water; acetone at 55 - 65℃; for 0.5h; Stage #2: In ethanol; water; acetone at 0 - 40℃; for 4h; | 90.3% |
-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

-
-
1043449-71-3
loxoprofen tolperisone salt

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 20℃; for 0.5h; |
-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water; toluene |
-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

-
-
81762-92-7
loxoprofen methyl ester

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogenchloride / water; toluene 2: dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / 0 - 20 °C View Scheme |
-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

-
-
89631-57-2, 89675-48-9, 90319-84-9, 90319-85-0, 90319-86-1, 90319-87-2
methyl (+/-)-2-<4-(trans-2-hydroxycyclopentylmethyl)phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrogenchloride / water; toluene 2: dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / 0 - 20 °C 3: sodium tetrahydroborate / ethanol / 2 h / 0 °C View Scheme |
-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydrogenchloride / water; toluene 2: dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / 0 - 20 °C 3: sodium tetrahydroborate / ethanol / 2 h / 0 °C 4: 1H-imidazole / dichloromethane; N,N-dimethyl-formamide View Scheme |
-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: hydrogenchloride / water; toluene 2: dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / 0 - 20 °C 3: sodium tetrahydroborate / ethanol / 2 h / 0 °C 4: 1H-imidazole / dichloromethane; N,N-dimethyl-formamide 5: lithium hydroxide monohydrate / water; tetrahydrofuran; ethanol View Scheme |
-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: hydrogenchloride / water; toluene 2: dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / 0 - 20 °C 3: sodium tetrahydroborate / ethanol / 2 h / 0 °C 4: 1H-imidazole / dichloromethane; N,N-dimethyl-formamide 5: lithium hydroxide monohydrate / water; tetrahydrofuran; ethanol 6: dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 0 - 20 °C View Scheme |

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| With triethylamine In toluene |
-
-
33657-49-7
chloromethyl n-butyrate

-
-
80382-23-6
sodium 2-<4-<(2-oxocyclopentyl)methyl>phenyl>propionate

| Conditions | Yield |
|---|---|
| With triethylamine In toluene |
Loxoprofen sodium Consensus Reports
Loxoprofen (CAS NO.80382-23-6) Market Research Report 2009
Loxoprofen sodium Specification
The Loxoprofen sodium, with the CAS registry number 80382-23-6, is also known as Benzeneaceticacid, a-methyl-4-[(2-oxocyclopentyl)methyl]-,sodium salt (9CI). It belongs to the product categories of APIS; Aromatics; Inhibitors; Intermediates & Fine Chemicals; Pharmaceuticals. This chemical's molecular formula is C15H17NaO3 and molecular weight is 268.28. What's more, its systematic name is Sodium 2-{4-[(2-oxocyclopentyl)methyl]phenyl}propanoate.
Physical properties about Loxoprofen sodium (CAS 80382-23-6) are: (1)ACD/LogP: 1.866; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.73; (4)ACD/LogD (pH 7.4): -1.07; (5)ACD/BCF (pH 5.5): 1.12; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 17.90; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 3; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 4; (12)Polar Surface Area: 54.37 Å2; (13)Flash Point: 220.7 °C; (14)Enthalpy of Vaporization: 70.78 kJ/mol; (15)Boiling Point: 417.9 °C at 760 mmHg; (16)Vapour Pressure: 9.92E-08 mmHg at 25°C.
Preparation of Loxoprofen sodium (CAS 80382-23-6): it can be produced by ethyl 2-oxocyclopentanecarboxylate (I) with ethyl 2-(4-chloromethylphenyl)propionate (II) by means of KOH in hot DMF. The reaction gives ethyl 2-[4-(1-ethoxycarbonyl-2-oxocyclopentan-1-ylmethyl)phenyl]propionate (III), which is then hydrolyzed and decarboxylated by treatment with 47% HBr in refluxing dioxane.
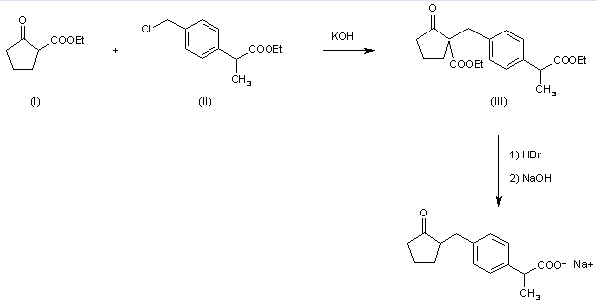
Uses of Loxoprofen sodium (CAS 80382-23-6): it is a non-steroidal anti-inflammatory drug which is used for chronic and wet arthritis, deformability arthropathy, waist pain and periarthritis of shoulder. It's the sodium salt of Loxoprofen marketed in Brazil, Mexico and Japan by Sankyo.
You can still convert the following datas of Loxoprofen sodium (CAS 80382-23-6) into molecular structure:
(1) SMILES:[Na+].O=C2C(Cc1ccc(cc1)C(C([O-])=O)C)CCC2
(2) Std. InChI:InChI=1S/C15H18O3.Na/c1-10(15(17)18)12-7-5-11(6-8-12)9-13-3-2-4-14(13)16;/h5-8,10,13H,2-4,9H2,1H3,(H,17,18);/q;+1/p-1
(3) Std. InChIKey:WORCCYVLMMTGFR-UHFFFAOYSA-M
Related Products
- Loxoprofen
- Loxoprofen sodium
- 80382-27-0
- 80382-29-2
- 80384-27-6
- 80-38-6
- 80387-96-8
- 80388-04-1
- 8038-90-2
- 8039-09-6
- 80-39-7
- 80-40-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View