-
Name
Myricetin
- EINECS 208-463-2
- CAS No. 529-44-2
- Article Data34
- CAS DataBase
- Density 1.912 g/cm3
- Solubility slightly soluble in water
- Melting Point >300 °C(lit.)
- Formula C15H10O8
- Boiling Point 747.6 °C at 760 mmHg
- Molecular Weight 318.24
- Flash Point 285.9 °C
- Transport Information
- Appearance brown-yellow to brown-green crystalline powder
- Safety 24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Flavone,3,3',4',5,5',7-hexahydroxy- (8CI);3,3',4',5,5',7-Hexahydroxyflavone;3,5,7,3',4',5'-Hexahydroxyflavone;Cannabiscetin;4H-1-benzopyran-4-one, 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-;3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphényl)-4H-chromén-4-one;
- PSA 151.59000
- LogP 1.69360
Synthetic route
-
-
14585-04-7
5,7-dihydroxy-3,3',4',5'-tetramethoxyflavone

-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at 10 - 15℃; for 10h; | 95.2% |
-
-
15648-86-9, 19833-12-6
isomyricitrin

-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| Acidic conditions; | 64% |

| Conditions | Yield |
|---|---|
| With sulfuric acid for 0.0666667h; Heating; | A n/a B 45% |
| With sulfuric acid for 0.0666667h; Heating; | A n/a B 45% |
-
-
27200-12-0
ampelopsin

-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| Stage #1: ampelopsin With sodium hydroxide In water for 0.25h; Reflux; Stage #2: With selenium(IV) oxide In water for 1.5h; Reagent/catalyst; Reflux; | 42% |
-
-
10034-85-2
hydrogen iodide

-
-
256486-48-3
2',6,6',8-tetrabromo-3,3',4',5,5',7-hexahydroxyflavone

-
-
529-44-2
myricetin

-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With hydrogen iodide; acetic anhydride |

-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With hydrogen iodide; phenol |

-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol for 4h; Heating; |

-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With hydrogen iodide; phenol at 145℃; for 0.5h; | 7 mg |
-
-
17912-87-7
myricitrin

-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With naringinase In acetate buffer at 20℃; for 3h; pH=5.5; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water for 0.25h; Heating; |
-
-
1241909-29-4
myricetin-3-O-β-D-galactoside-3'-O-α-D-glucoside

-
A
-
2280-44-6
D-Glucose

-
B
-
10257-28-0
D-Galactose

-
C
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol at 90℃; for 2h; |

-
-
1241909-30-7
myricetin-3-O-β-D-galactoside-3'-O-α-D-galactoside

-
A
-
10257-28-0
D-Galactose

-
B
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol at 90℃; for 2h; |
-
-
100-53-8
phenylmethanethiol

-
A
-
220886-23-7
(epi)gallocatechin-3-O-gallate benzylthioether

-
B
-
529-44-2
myricetin

-
C
-
213007-61-5
(-)-epicatechin benzylthioether

-
D
-
220886-22-6
epigallocatechin-4-benzylthioether


-
F
-
2596-50-1, 4233-96-9, 5127-64-0, 19065-20-4, 107965-88-8, 137766-94-0, 989-51-5
(-)-epigallocathechin gallate

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 60℃; for 2h; |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In methanol at 100℃; for 2h; | |
| Acidic conditions; |
-
-
1357272-88-8
myricetin 3-O-(2'',3''-digalloyl)-β-D-galactopyranoside

-
A
-
59-23-4
D-Galactose

-
B
-
149-91-7
3,4,5-trihydroxybenzoic acid

-
C
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| Stage #1: myricetin 3-O-(2'',3''-digalloyl)-β-D-galactopyranoside With sulfuric acid; water at 85℃; for 3h; Stage #2: In water |

-
-
557792-97-9
ampelopsin

-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| In water at 100℃; for 12h; |
-
-
480-18-2
taxifolin

-
A
-
99-50-3
3,4-Dihydroxybenzoic acid

-
B
-
83-30-7
acide 2,4,6-trihydroxybenzoique

-
D
-
30048-34-1
2-(3',4'-dihydroxybenzoyloxy)-4,6-dihydroxybenzoic acid

-
F
-
69098-01-7
2-oxo-2-(2,4,6-trihydroxyphenyl)acetic acid

-
G
-
529-44-2
myricetin

-
H
-
139-85-5
3,4-dihydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With oxygen; potassium hydroxide In methanol |


-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With acetic acid In water at 100℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 100℃; for 2h; |

| Conditions | Yield |
|---|---|
| Acidic conditions; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrogenchloride / ethyl acetate / 7 h / 20 °C 2: triethylamine / 6 h / 90 °C 3: boron tribromide / dichloromethane / 10 h / 10 - 15 °C View Scheme |

| Conditions | Yield |
|---|---|
| With sulfuric acid In water for 4h; |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 20℃; for 12h; | 95% |
| With potassium carbonate In acetone for 14h; Heating; |

| Conditions | Yield |
|---|---|
| Stage #1: procaine hydrochloride With hydrogenchloride; sodium acetate; sodium nitrite In water Diazotization; Stage #2: myricetin In ethanol; water at 25℃; Substitution; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-aminobenzene sulfonic acid With sodium hydroxide; sodium nitrite In water at 0℃; for 1h; Diazotization; Stage #2: myricetin In ethanol; water at 25℃; for 1h; Substitution; Stage #3: With sodium chloride In ethanol; water at 0℃; for 3h; Sodium salt formation; | 95% |
-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| In ethanol; acetic acid at 90 - 95℃; for 5h; Condensation; | 93% |
-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In ethanol at 60℃; for 1.5h; Ring cleavage; | 86% |
-
-
529-44-2
myricetin

-
-
108-24-7
acetic anhydride

-
-
14813-29-7
[3,5-diacetoxy-4-oxo-2-(3,4,5-triacetoxyphenyl)chromen-7-yl] acetate

| Conditions | Yield |
|---|---|
| With sodium acetate at 80℃; for 6h; | 85% |
| With pyridine at 15℃; for 16h; | 61% |
| With pyridine at 15℃; for 12h; | 61% |

| Conditions | Yield |
|---|---|
| Stage #1: Sulfathiazole With hydrogenchloride; sodium acetate; sodium nitrite In water Diazotization; Stage #2: myricetin In ethanol; water at 25℃; Substitution; | 82% |

| Conditions | Yield |
|---|---|
| Stage #1: sulfadimesine With hydrogenchloride; sodium acetate; sodium nitrite In water Diazotization; Stage #2: myricetin In ethanol; water at 25℃; Substitution; | 81% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 12h; Inert atmosphere; | 79.79% |

| Conditions | Yield |
|---|---|
| With pyridine for 12h; Inert atmosphere; | 79.79% |
-
-
529-44-2
myricetin

-
-
256486-45-0
3,3',4',5,5',7-hexahydroxy-2',6,6',8-tetranitroflavone

| Conditions | Yield |
|---|---|
| With nitric acid In acetic acid at 20℃; for 3h; Nitration; | 77% |
-
-
529-44-2
myricetin

-
-
256486-48-3
2',6,6',8-tetrabromo-3,3',4',5,5',7-hexahydroxyflavone

| Conditions | Yield |
|---|---|
| With bromine In acetic acid at 20℃; for 3h; Bromination; | 75% |
| With carbon disulfide; bromine at 100℃; |

| Conditions | Yield |
|---|---|
| Stage #1: sulfanilamide With hydrogenchloride; sodium nitrite In water Diazotization; Stage #2: myricetin In ethanol; water at 23 - 25℃; Substitution; | 73% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-nitro-aniline With hydrogenchloride; sodium nitrite In water at 0 - 5℃; Diazotization; Stage #2: myricetin In ethanol; water at 25℃; Substitution; | 70% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-benzyloxybenzoyl chloride; myricetin In acetonitrile at 20℃; for 0.5h; Stage #2: With dmap at 20℃; for 12h; | 63.4% |
-
-
529-44-2
myricetin

-
-
74-88-4
methyl iodide

-
-
190323-49-0
3-hydroxy-5,7-dimethoxy-2–(3,4,5-trimethoxyphenyl)-4H-chromen-4-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 48h; | 54.4% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 60h; | |
| Stage #1: myricetin With potassium carbonate In N,N-dimethyl-formamide at 20℃; Stage #2: methyl iodide In N,N-dimethyl-formamide at 20℃; for 48h; | |
| Stage #1: myricetin With 2K(1+)*CO3(2-)*0.5H2O=K2CO3*0.5H2O In N,N-dimethyl-formamide at 20℃; Stage #2: methyl iodide In N,N-dimethyl-formamide at 20℃; for 48h; | |
| Stage #1: myricetin With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: methyl iodide In N,N-dimethyl-formamide at 20℃; for 48h; |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 20℃; for 312h; | 54% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide Williamson Ether Synthesis; | 52% |
-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With 2,3-dibromo-3-phenylpropanoic acid; caesium carbonate In N,N-dimethyl-formamide at 40℃; for 16h; Inert atmosphere; regioselective reaction; | 49% |

| Conditions | Yield |
|---|---|
| Stage #1: myricetin With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 1h; Inert atmosphere; Stage #2: benzyl bromide at 70℃; for 12h; | 46% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide Williamson Ether Synthesis; | 45% |
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; | 262 mg |
-
-
16695-22-0
N,N-bis(tosyloxyethyl)-p-toluenesulfonamide

-
-
529-44-2
myricetin

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide Williamson Ether Synthesis; | 45% |
| With potassium carbonate In N,N-dimethyl-formamide at 110℃; | 402 g |

| Conditions | Yield |
|---|---|
| With pyridine at 85℃; for 24h; | A 45% B 18% C 16% |


| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide Williamson Ether Synthesis; | 36% |
| With potassium carbonate In N,N-dimethyl-formamide at 110℃; | 236 mg |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
529-44-2
myricetin

-
A
-
16280-27-6
3,5,3',4',5'-pentahydroxy-7-methoxyflavone

-
B
-
16805-10-0
3,5,7-trihydroxy-2-(3,5-dihydroxy-4-methoxyphenyl)-4H-chromen-4-one

| Conditions | Yield |
|---|---|
| In methanol; diethyl ether at 20℃; for 1h; | A 25% B 31% C 9% D 6% |


| Conditions | Yield |
|---|---|
| With pyridine at 0 - 20℃; Inert atmosphere; | 30% |
| With pyridine at 0 - 20℃; Inert atmosphere; | 30% |
| With pyridine at 0℃; Inert atmosphere; | 30% |
| With pyridine at 0 - 20℃; Inert atmosphere; | 30% |
Myricetin Chemical Properties
Molecular Structure:
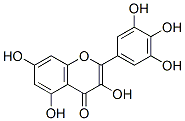
IUPAC Name:3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one
Molecular Formula:C15H10O8
Molecular Weight:318.2351
H bond acceptors: 8
H bond donors: 6
Freely Rotating Bonds: 7
Polar Surface Area: 81.68 Å2
Molar Refractivity: 75.2 cm3
Molar Volume: 166.4 cm3
Surface Tension: 133 dyne/cm
Density: 1.912 g/cm3
Melting Point: >300°C (lit.)
Boiling Point: 747.6°C at 760 mmHg
Flash Point: 285.9°C
Index of Refraction: 1.863
Form: crystalline
Merck: 13,6357
EINECS: 208-463-2
Product Categories: Flavanols; Nutritional Ingredients
InChI
InChI=1/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H
Smiles
c12c(c(c(O)c(o1)c1cc(c(O)c(c1)O)O)=O)c(cc(c2)O)O
Myricetin Uses
Myricetin (CAS NO.529-44-2) is widely used in the fields of medicine, food, health care products and cosmetics. Myricetin (CAS NO.529-44-2) is mainly used as a additive to treat and prevent arthritis, especially for pregnant or lactating women. Myricetin (CAS NO.529-44-2) is favorable to decrease the side effects caused by antibiotics.
Myricetin Toxicity Data With Reference
| 1. |
| PYTCAS Phytochemistry. An International Journal of Plant Biochemistry. 26 (1987),2231. | ||
| 2. | dnd-hmn-hla 400 µmol/L | MUREAV Mutation Research. 390 (1997),141. | ||
| 3. | dni-hmn-hla 12500 µg/L | PYTCAS Phytochemistry. An International Journal of Plant Biochemistry. 27 (1988),1017. EXPEAM Experientia. 44 (1988),882. |
Myricetin Safety Profile
Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating vapors.
Safety Statements:24/25
24/25:Avoid contact with skin and eyes
RTECS: LK8646000
F: 10
WGK Germany: 3
Myricetin Specification
Myricetin , with CAS number of 529-44-2, can be called 3,3’,4’,5,5’,7-hexahydroxy-flavon ; 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4h-1-benzopyran-4-on ; Cannabiscetin ; 3,3',4',5,5',7-hexahydroxyflavone ; 3',4',5',3,5,7-hexahydroxyflavone ; 3,5,7,3',4',5'-hexahydroxyflavone ; Myricetin ; Myricetol . Myricetin (CAS NO.529-44-2) is a compoud that belongs to flavonols. It is incompatibilities with strong oxidizing agents. It emits carbon monoxide and carbon dioxide when it is decomposed. Myricetin (CAS NO.529-44-2) belongs to flavonols. Myricetin (CAS NO.529-44-2) exists as a brown-yellow to brown-green crystalline powder, soluble in methanol, ethanol, acetone, ethyl acetate, slightly soluble in water, insoluble in chloroform, petroleum ether. Myricetin (CAS NO.529-44-2) is easily turns green in the air.
Related Products
- Myricetin
- Myricetin hexaacetate
- 52944-66-8
- 5294-61-1
- 52946-22-2
- 529-49-7
- 52950-18-2
- 52950-19-3
- 529509-58-8
- 529512-80-9
- 529-53-3
- 529-59-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View