-
Name
N-Boc-4-piperidinemethanol
- EINECS
- CAS No. 123855-51-6
- Article Data165
- CAS DataBase
- Density 1.059 g/cm3
- Solubility
- Melting Point 78-82 °C
- Formula C11H21NO3
- Boiling Point 308 °C at 760 mmHg
- Molecular Weight 215.293
- Flash Point 140.1 °C
- Transport Information
- Appearance
- Safety 24/25
- Risk Codes
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 4-(Hydroxymethyl)-1-piperidinecarboxylic acid1,1-dimethylethyl ester;4-(Hydroxymethyl)piperidine-1-carboxylic acidtert-butyl ester;4-Hydroxymethylpiperidine-1-carboxylic acid tert-butyl ester;N-(tert-Butoxycarbonyl)-4-piperidinemethanol;N-BOC-piperidine-4-methanol;1-(tert-Butoxycarbonyl)-4-(hydroxymethyl)piperidine;1-(tert-Butoxycarbonyl)-4-piperidinemethanol;1-Boc-4-Hydroxymethylpiperidine;
- PSA 49.77000
- LogP 1.56370
Synthetic route
-
-
6457-49-4
4-piperidinemethanol

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane | 100% |
| In tetrahydrofuran; ethyl acetate at 20℃; for 14h; | 100% |
| With sodium carbonate In tetrahydrofuran; water at 95℃; for 2.5h; | 100% |
-
-
84358-13-4
N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With borane-THF In tetrahydrofuran | 100% |
| Stage #1: N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid With borane-THF In tetrahydrofuran at 0℃; for 0.5h; Stage #2: With methanol | 96% |
| With borane-THF In tetrahydrofuran at 0℃; for 0.666667h; Inert atmosphere; | 89% |
-
-
137076-22-3
tert butyl 4-formylpiperidine-1-carboxylate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With methanol; sodium tetrahydroborate at 0 - 25℃; for 1h; | 100% |
| With sodium tetrahydroborate In ethanol for 1h; Inert atmosphere; | 53% |
-
-
142851-03-4
1-tert-butyl 4-ethyl piperidine-1,4-dicarboxylate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 1-tert-butyl 4-ethyl piperidine-1,4-dicarboxylate With diisobutylaluminium hydride In tetrahydrofuran at -78 - 20℃; for 0.833333h; Stage #2: With ammonium chloride In tetrahydrofuran; water at 20℃; for 0.75h; | 97% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 2h; | 96% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; | 94% |
-
-
145508-94-7
tert-butyl 4-(iodomethyl)piperidine-1-carboxylate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In tetrahydrofuran | 93% |
-
-
541-41-3
chloroformic acid ethyl ester

-
-
84358-13-4
N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; sodium borohydrid In tetrahydrofuran; methanol; ethyl acetate | 90% |
| With 4-methyl-morpholine; sodium borohydrid In tetrahydrofuran; methanol; ethyl acetate | 90% |

-
-
584-08-7
potassium carbonate

-
-
84358-13-4
N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water | 84% |

-
-
584-08-7
potassium carbonate

-
-
84358-13-4
N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water | 84% |
-
-
124443-68-1
1-tert-Butyl 4-methyl piperidine-1,4-dicarboxylate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 1-tert-Butyl 4-methyl piperidine-1,4-dicarboxylate With lithium borohydride In tetrahydrofuran at 0℃; Reflux; Stage #2: With water In tetrahydrofuran Product distribution / selectivity; | 82% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; | |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; | |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; | |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; Inert atmosphere; |
-
-
6457-49-4
4-piperidinemethanol

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Acylation; |
-
-
498-94-2
isonipecotic acid

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 92 percent / aq. NaOH, Et3N / dioxane / 20 h / Ambient temperature 2: BH3*THF / tetrahydrofuran / 1 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 100 percent / CH2Cl2 2: 100 percent / BH3*THF / tetrahydrofuran View Scheme | |
| Multi-step reaction with 2 steps 1: 91 percent / NaHCO3 / dioxane; H2O / 24 h 2: 84 percent / BH3*THF / tetrahydrofuran / 0 - 25 °C View Scheme |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 92 percent / aq. NaOH, Et3N / dioxane / 20 h / Ambient temperature 2: BH3*THF / tetrahydrofuran / 1 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 100 percent / CH2Cl2 2: 100 percent / BH3*THF / tetrahydrofuran View Scheme | |
| Multi-step reaction with 2 steps 1: 91 percent / NaHCO3 / dioxane; H2O / 24 h 2: 84 percent / BH3*THF / tetrahydrofuran / 0 - 25 °C View Scheme |
-
-
1126-09-6
4-carbethoxypiperidine

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 98 percent / Et3N; DMAP / CH2Cl2 2: 94 percent / LiAlH4 / tetrahydrofuran / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 99 percent / ethyl acetate / 20 h / Ambient temperature 2: 84 percent / LAH / tetrahydrofuran / 2 h / 0 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 94 percent / Et3N / dioxane; H2O / 16 h / 0 - 20 °C 2: 93 percent / LiAlH4 / tetrahydrofuran / 16 h / 0 - 20 °C View Scheme |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 98 percent / Et3N; DMAP / CH2Cl2 2: 94 percent / LiAlH4 / tetrahydrofuran / 0 °C View Scheme |
-
-
498-94-2
isonipecotic acid

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / NaHCO3 / dioxane; H2O / 24 h 2: 84 percent / BH3*THF / 0 - 25 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: aq. NaOH / 1,2-dimethoxy-ethane 2.1: isobutyl chloroformate; N-methylmorpholine / 1,2-dimethoxy-ethane / 0 °C 2.2: aq. NaBH4 View Scheme |
-
-
24424-99-5
di-tert-butyl dicarbonate


-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 91 percent / NaHCO3 / dioxane; H2O / 24 h 2: 84 percent / BH3*THF / 0 - 25 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: aq. NaOH / 1,2-dimethoxy-ethane 2.1: isobutyl chloroformate; N-methylmorpholine / 1,2-dimethoxy-ethane / 0 °C 2.2: aq. NaBH4 View Scheme |
-
-
6457-49-4
4-piperidinemethanol

-
-
34619-03-9
tert-butyldicarbonate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 12h; | |
| In dichloromethane at 0 - 20℃; |
-
-
14044-65-6
borane-THF

-
-
84358-13-4
N-[(tert-butoxy)carbonyl]piperidine-4-carboxylic acid

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran |
-
-
1126-09-6
4-carbethoxypiperidine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
142851-03-4
1-tert-butyl 4-ethyl piperidine-1,4-dicarboxylate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With sodium carbonate In 1,4-dioxane; diethyl ether; ethyl acetate | 11.7 gm (36%) |

-
-
498-94-2
isonipecotic acid

-
-
6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-
-
141-78-6
ethyl acetate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate; triethylamine In tetrahydrofuran; 1,4-dioxane; hexane; dichloromethane |


-
-
137076-22-3
tert butyl 4-formylpiperidine-1-carboxylate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; hexane |
-
-
1401098-21-2
tert-butyl 4-((4-(benzyloxy)phenoxy)methyl)piperidine-1-carboxylate

-
A
-
1401098-22-3
tert-butyl 4-((4-hydroxycyclohexyloxy)methyl)piperidine-1-carboxylate

-
B
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrogen / palladium 10% on activated carbon / ethanol; water / 15 h / 23 °C / 760.05 Torr 2: hydrogen; sodium hydroxide / rhodium contaminated with carbon / water / 21 h / 60 - 70 °C / 7500.75 - 9750.98 Torr View Scheme |
-
-
1350060-40-0
tert-butyl 4-[(4-hydroxyphenoxy)methyl]piperidine-1-carboxylate

-
A
-
1401098-22-3
tert-butyl 4-((4-hydroxycyclohexyloxy)methyl)piperidine-1-carboxylate

-
B
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With hydrogen; sodium hydroxide; rhodium contaminated with carbon In water at 60 - 70℃; under 7500.75 - 9750.98 Torr; for 21h; |

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine / dichloromethane / 16 h / 20 °C 2: lithium aluminium tetrahydride / tetrahydrofuran / 6 h / 0 - 45 °C View Scheme |
-
-
79099-07-3
N-tert-butyloxycarbonylpiperidin-4-one

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium hexamethyldisilazane / tetrahydrofuran / 1 h / 0 °C 1.2: 12 h / 0 - 25 °C 2.1: cerium(III) chloride; sodium iodide / acetonitrile / 12 h / Reflux 3.1: sodium tetrahydroborate; methanol / 1 h / 0 - 25 °C View Scheme |
-
-
138022-91-0
tert-butyl 4-(methoxymethylene)piperidine-1-carboxylate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: cerium(III) chloride; sodium iodide / acetonitrile / 12 h / Reflux 2: sodium tetrahydroborate; methanol / 1 h / 0 - 25 °C View Scheme |
-
-
2971-79-1
isonipecotic acid methyl ester

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine / dichloromethane / 12 h / 25 °C 2: lithium borohydride / tetrahydrofuran / 0 °C / Reflux View Scheme |
-
-
55-22-1
pyridine-4-carboxylic acid

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: hydrogen / water / 1.5 h / 80 - 95 °C 2: potassium hydroxide; water / ethanol 3: sodium tetrahydroborate / tetrahydrofuran / 3.5 h / -5 - 0 °C View Scheme |
-
-
124-63-0
methanesulfonyl chloride

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
161975-39-9
tert-butyl 4-(((methylsulfonyl)oxy)methyl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 5℃; for 1.5h; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 1.33333h; | 100% |
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; | 100% |
-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
137076-22-3
tert butyl 4-formylpiperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With oxalyl dichloride; dimethyl sulfoxide; triethylamine In dichloromethane Swern oxidation; | 100% |
| Stage #1: tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate With oxalyl dichloride; dimethyl sulfoxide In dichloromethane for 0.25h; Cooling with acetone-dry ice; Stage #2: With triethylamine In dichloromethane at 20℃; | 100% |
| Stage #1: tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate With oxalyl dichloride; dimethyl sulfoxide In dichloromethane at -20℃; for 0.333333h; Swern oxidation; Inert atmosphere; Stage #2: With triethylamine In dichloromethane at -50 - 20℃; Swern oxidation; Inert atmosphere; | 100% |
-
-
100-39-0
benzyl bromide

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
301226-04-0
tert-butyl 4-((benzyloxy)methyl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With sodium hydride In DMF (N,N-dimethyl-formamide) at 0 - 20℃; for 23h; | 100% |
| Stage #1: tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate With sodium hydride In tetrahydrofuran; mineral oil at 0℃; for 0.25h; Inert atmosphere; Stage #2: benzyl bromide In tetrahydrofuran; mineral oil at 0 - 80℃; for 3h; Inert atmosphere; | 76% |
| Stage #1: tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate With sodium hydride In DMF (N,N-dimethyl-formamide) at 0 - 20℃; for 0.75h; Stage #2: benzyl bromide In DMF (N,N-dimethyl-formamide) at 0 - 60℃; | |
| With NaH In hexane; N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| Stage #1: C13H12N4O3; tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate With PS-triphenylphosphine In dichloromethane for 0.0833333h; Stage #2: With di-tert-butyl-diazodicarboxylate In dichloromethane at 20℃; for 3h; Stage #3: With trifluoroacetic acid In dichloromethane | 100% |
-
-
124-63-0
methanesulfonyl chloride

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran; water | 100% |
-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
479057-79-9
tert-butyl 4-(chloromethyl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With tetrachloromethane In dichloromethane at 20℃; | 100% |
-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 20℃; for 3h; | 100% |
| With hydrogenchloride In 1,4-dioxane; dichloromethane at 20℃; for 6h; | 77% |
| With hydrogenchloride In methanol; diethyl ether for 1h; | |
| With hydrogenchloride In 1,4-dioxane; water for 1h; |
-
-
124-63-0
methanesulfonyl chloride

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
194872-09-8
4-(methylsulfonylmethyl)piperidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 12.5h; | 100% |
-
-
98-59-9
p-toluenesulfonyl chloride

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
166815-96-9
N-tert-butoxycarbonyl-4-(4-toluenesulphonyloxymethyl)piperidine

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0 - 17℃; for 2h; Inert atmosphere; | 99% |
| With triethylamine In dichloromethane at 10 - 20℃; for 12h; | 92.1% |
| With pyridine at 0 - 20℃; for 16h; | 91% |
-
-
42098-25-9
1-methyl-4-nitro-5-chloro-1H-pyrazole

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
1338717-88-6
tert-butyl 4-((4-amino-1-methyl-1H-pyrazol-5-yloxy)methyl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 5-chloro-1-methyl-4-nitro-1H-pyrazole; tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate With sodium hydride In N,N-dimethyl-formamide for 1h; Stage #2: With iron; ammonium chloride In ethanol; water at 60℃; for 1h; | 99% |
-
-
624-28-2
2,5-dibromopyridine

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
1010114-48-3
tert-butyl 4-((5-bromopyridin-2-yloxy)methyl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 20℃; for 3h; Temperature; Concentration; | 98% |
| With sodium hydride In N,N-dimethyl-formamide; mineral oil at 0 - 80℃; for 5h; | 87% |
| Stage #1: tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate With sodium hydride In dimethyl sulfoxide; mineral oil at 20 - 50℃; for 1.5h; Stage #2: 2,5-dibromopyridine In dimethyl sulfoxide; mineral oil at 20℃; | 84% |
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 3h; | 78% |

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In dichloromethane at 20℃; for 18h; Inert atmosphere; | 98% |
-
-
881902-80-3
4,6-dichloro-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-pyrrolo[2,3-b]pyridine

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate With sodium hydride In dimethyl sulfoxide; mineral oil at 20℃; for 0.5h; Stage #2: 4,6-dichloro-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-pyrrolo[2,3-b]pyridine In dimethyl sulfoxide; mineral oil at 20℃; for 2h; | 98% |
-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
145508-94-7
tert-butyl 4-(iodomethyl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With 1H-imidazole; iodine; triphenylphosphine In tetrahydrofuran at 20℃; | 97% |
| With pyridine; iodine; triphenylphosphine In benzene for 1.5h; Heating; | 92% |
| With 1H-imidazole; iodine; triphenylphosphine In tetrahydrofuran at 20℃; for 14h; | 92% |
-
-
62810-39-3
(4-chlorophenyl)(3-hydroxyphenyl)methanone

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
1372526-57-2
tert-butyl 4-{[3-(4-chlorobenzoyl)phenoxy]methyl}-piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran for 3h; Mitsunobu reaction; | 97% |
-
-
7693-46-1
4-Nitrophenyl chloroformate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
1159928-33-2
(1-(tert-Butoxycarbonyl)piperidin-4-yl)methyl 4-nitrophenyl carbonate

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine In DCM at 0 - 20℃; | 96% |
-
-
1806-29-7
2,2'-dihydroxybiphenyl

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 6h; Inert atmosphere; | 96% |
-
-
130115-85-4
5-bromo-6-chloropyridin-3-ol

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0 - 20℃; for 5h; | 96% |
-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
158407-04-6
tert-butyl 4-(bromomethyl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In dichloromethane at 20℃; for 1h; | 95% |
| With carbon tetrabromide; triphenylphosphine In dichloromethane for 0.166667h; | 95% |
| With carbon tetrabromide; triphenylphosphine In dichloromethane at 0 - 20℃; for 22h; | 94% |

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In dichloromethane at 0 - 20℃; for 28h; Inert atmosphere; | 95% |

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6; [D3]acetonitrile at 40℃; for 15h; Inert atmosphere; | 95% |
-
-
1112181-71-1
N-(3-methoxybenzyl)-6-methyl-4-(2H-tetrazol-5-yl)picolinamide

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With di-tert-butyl-diazodicarboxylate In tetrahydrofuran at 0 - 20℃; for 15h; | 94% |
-
-
524-38-9
N-hydroxyphthalimide

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

-
-
143540-03-8
N-(N-Boc-piperidin-4-yl)methoxyphthalimide

| Conditions | Yield |
|---|---|
| With triphenylphosphine; diethylazodicarboxylate In tetrahydrofuran at 0 - 20℃; for 4h; Inert atmosphere; | 94% |
-
-
36953-42-1
3-bromo-4-chloropyridine

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide | 94% |
-
-
570407-88-4
tert-butyl 3-chloro-4-fluorobenzoate

-
-
123855-51-6
tert-butyl 4-(hydroxymethyl)piperidin-1-carboxylate

| Conditions | Yield |
|---|---|
| With caesium carbonate In dimethyl sulfoxide at 80℃; for 18h; Inert atmosphere; | 94% |
N-Boc-4-piperidinemethanol Chemical Properties
IUPAC Name: tert-Butyl 4-(hydroxymethyl)piperidine-1-carboxylate
Synonyms of N-Boc-4-piperidinemethanol (CAS NO.123855-51-6): N-tert-Butyloxycarbonyl-4-piperidinemethanol
CAS NO: 123855-51-6
Molecular formula: C11H21NO3
Molecular Weight: 215.29
Molecular Structure : 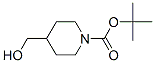
Melting point:78-82 °C(lit.)
H bond acceptors: 4
H bond donors: 1
Freely Rotating Bonds: 4
Polar Surface Area: 38.77 Å2
Index of Refraction: 1.479
Molar Refractivity: 57.66 cm3
Molar Volume: 203.2 cm3
Surface Tension: 37.8 dyne/cm
Density: 1.059 g/cm3
Flash Point: 140.1 °C
Enthalpy of Vaporization: 63.62 kJ/mol
Boiling Point: 308 °C at 760 mmHg
Vapour Pressure: 6.41E-05 mmHg at 25°C
Melting Point: 78-82 °C(lit.)
Product Categories of N-Boc-4-piperidinemethanol (CAS NO.123855-51-6): Amines;blocks;Piperidine;Piperazine
N-Boc-4-piperidinemethanol Safety Profile
Safety Information about N-Boc-4-piperidinemethanol (CAS NO.123855-51-6):
Hazard Codes:  Xi
Xi
WGK Germany: 3
Hazard Note: Irritant
Related Products
- N-Boc-4-piperidinemethanol
- 123855-55-0
- 123-86-4
- 123864-74-4
- 1238673-32-9
- 123871-90-9
- 12389-15-0
- 123891-64-5
- 123895-42-1
- 12389-75-2
- 123-90-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View