-
Name
N,N-Dimethylaniline
- EINECS 204-493-5
- CAS No. 121-69-7
- Article Data664
- CAS DataBase
- Density 1.038 g/mL at 25 °C
- Solubility Slightly soluble in water, volatilize with water vapor. Miscible with alcohol, ether, chloroform, benzene and many kinds of organic solvents.
- Melting Point 1100 °C(lit.)
- Formula C8H11N
- Boiling Point 193.539 °C at 760 mmHg
- Molecular Weight 121.182
- Flash Point 62.778 °C
- Transport Information UN 2253 6.1/PG 2
- Appearance A yellow to brown colored oily liquid with a fishlike odor
- Safety 53-45-61-36/37-28
- Risk Codes 61-20/21-51/53-40-23/24/25
-
Molecular Structure
-
Hazard Symbols
 T,
T, N
N
- Synonyms Aniline,N,N-dimethyl- (8CI);(Dimethylamino)benzene;Dimethylaniline;Dimethylphenylamine;EP 210;N,N-Dimethylaminobenzene;N,N-Dimethylbenzenamine;N,N-Dimethylphenylamine;NSC 7195;Versneller NL 63/10;Benzenamine,N,N-dimethyl-;
- PSA 3.24000
- LogP 1.75260
Synthetic route

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine; tetrahydroxydiboron; 5%-palladium/activated carbon In 1,2-dichloro-ethane at 50℃; for 3h; | 100% |
| With lithium aluminium tetrahydride; di-tert-butyl peroxide In tetrahydrofuran for 3h; Irradiation; | 93% |
| With isopropyl alcohol at 20℃; for 24h; UV-irradiation; chemoselective reaction; | 82% |

| Conditions | Yield |
|---|---|
| With 9-borabicyclo[3.3.1]nonane dimer; proazaphosphatrane In tetrahydrofuran-d8 at 90℃; under 750.075 Torr; for 0.166667h; Catalytic behavior; Time; Inert atmosphere; Schlenk technique; Sealed tube; chemoselective reaction; | 100% |
| With [Ru(Triphos)(TMM)]; hydrogen; bis(trifluoromethanesulfonyl)amide In tetrahydrofuran at 150℃; under 60006 Torr; for 10h; Temperature; Time; Reagent/catalyst; Pressure; Inert atmosphere; | 99% |
| With phenylsilane; triphenylphosphine In tetrahydrofuran at 120℃; under 3750.38 Torr; for 24h; Catalytic behavior; Reagent/catalyst; Autoclave; Green chemistry; | 99% |
-
-
171364-78-6
(4-(N,N-dimethylamino)phenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
121-69-7
N,N-dimethyl-aniline

| Conditions | Yield |
|---|---|
| With water In dimethylsulfoxide-d6 at 100℃; for 96h; | 100% |

-
-
121-69-7
N,N-dimethyl-aniline

| Conditions | Yield |
|---|---|
| With water In dimethylsulfoxide-d6 at 100℃; for 168h; | 100% |
-
-
124-38-9
carbon dioxide

-
-
1145-27-3
N,N’-dimethyl-N,N’-diphenylmethanediamine

-
-
121-69-7
N,N-dimethyl-aniline

| Conditions | Yield |
|---|---|
| With potassium tungstate; phenylsilane In acetonitrile at 70℃; for 12h; | 100% |

| Conditions | Yield |
|---|---|
| With ferric(III) bromide; 1,2,3,4,5-pentamethylcyclopentadiene; Pyroglutamic acid In 1,2,4-Trimethylbenzene at 200℃; for 36h; Inert atmosphere; | 99% |
| With tetrachloromethane; copper(ll) bromide at 180℃; for 6h; Reagent/catalyst; Temperature; Inert atmosphere; Sealed tube; | 99% |
| With 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine In 1,2,4-Trimethylbenzene at 200℃; for 36h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With tris(pentafluorophenyl)borate In dibutyl ether at 100℃; for 8h; Inert atmosphere; Schlenk technique; | 99% |
| With platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane complex; 1,3-bis-(diphenylphosphino)propane; phenylsilane In dibutyl ether at 20℃; for 18h; Schlenk technique; Inert atmosphere; | 98% |
| With tetrabutyl ammonium fluoride; HSiPh3 In acetonitrile at 50℃; | 95% |
-
-
98-04-4
phenyltrimethylammonium iodide

-
-
93296-10-7
(4-(dimethylamino)phenyl)zinc(II) chloride

-
A
-
1137-79-7
N,N-dimethyl-4-biphenylamine

-
B
-
121-69-7
N,N-dimethyl-aniline

| Conditions | Yield |
|---|---|
| With bis(tricyclohexylphosphine)nickel(II) dichloride In tetrahydrofuran; 1-methyl-pyrrolidin-2-one at 90℃; for 8h; Negishi coupling reaction; Inert atmosphere; | A 99% B n/a |


| Conditions | Yield |
|---|---|
| With NHC-Pd(II)-Im; potassium tert-butylate at 20℃; for 6h; Inert atmosphere; | 99% |
| With bis(1,5-cyclooctadiene)nickel (0); 7,9-bis(2,6-diisopropylphenyl)-7H-acenaphtho[1,2-d]imidazol-9-ium chloride; potassium tert-butylate In water; toluene at 35℃; for 24h; Catalytic behavior; Reagent/catalyst; Glovebox; Sealed tube; Inert atmosphere; | 99 %Chromat. |

| Conditions | Yield |
|---|---|
| With C27H36Cl2N2Zn at 100℃; for 20h; | 99% |

| Conditions | Yield |
|---|---|
| With phenylsilane In dibutyl ether at 100℃; for 8h; Catalytic behavior; Solvent; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 99% |
| With phenylsilane; copper diacetate In dibutyl ether at 80℃; for 8h; Catalytic behavior; Reagent/catalyst; Schlenk technique; Green chemistry; | 97% |
| With copper (II) carbonate hydroxide; phenylsilane; 1,4-di(diphenylphosphino)-butane In acetonitrile at 60℃; for 12h; | 74% |

| Conditions | Yield |
|---|---|
| With phenylsilane; triphenylphosphine In tetrahydrofuran at 120℃; under 3750.38 Torr; for 24h; Autoclave; Green chemistry; | 99% |
-
-
124-38-9
carbon dioxide

-
-
1145-27-3
N,N’-dimethyl-N,N’-diphenylmethanediamine

-
A
-
121-69-7
N,N-dimethyl-aniline

-
B
-
100-61-8
N-methylaniline

| Conditions | Yield |
|---|---|
| With diphenylsilane; cesium formate In acetonitrile at 50℃; under 750.075 Torr; Green chemistry; | A 99% B n/a |


| Conditions | Yield |
|---|---|
| With phenylboronic acid In dichloromethane at 20℃; for 0.0833333h; | 98% |
| With AFA In dichloromethane at 0 - 21℃; | 97% |
| With Amberlite IRA-400; borohydride form; copper(II) sulfate In methanol at 20℃; for 1h; Reduction; | 94% |

| Conditions | Yield |
|---|---|
| palladium at 120℃; for 20h; | A 98% B 98% |

| Conditions | Yield |
|---|---|
| With Raney-Ni at 169.84℃; under 22502.3 Torr; for 5h; Inert atmosphere; Autoclave; | 98% |
| With aluminum (III) chloride; water In acetonitrile at 20℃; Reagent/catalyst; Irradiation; | 88% |
| With TiO2 supported nano-Pd(0.8) catalyst In water at 20℃; for 15h; Inert atmosphere; Irradiation; Green chemistry; | 76 %Chromat. |
| With palladium 10% on activated carbon; potassium tert-butylate at 150℃; for 36h; |

| Conditions | Yield |
|---|---|
| With sodium carbonate In water; dimethyl sulfoxide at 130℃; for 15h; Schlenk technique; Sealed tube; Green chemistry; | 98% |
| With methanol; hydrogen at 79.84℃; under 9750.98 Torr; for 1.41667h; Autoclave; |

| Conditions | Yield |
|---|---|
| With disodium telluride In ethanol Heating; | 97% |
| With hydrogenchloride bei der Destillation; | |
| With potassium hydroxide | |
| Multi-step reaction with 2 steps 1: silver oxide 2: Erhitzen View Scheme |
-
-
110698-86-7
benzyl<2-(N,N-dimethylamino)phenyl>diphenylphosphonium bromide

-
A
-
2959-74-2
benzyldiphenylphosphine oxide

-
B
-
121-69-7
N,N-dimethyl-aniline

-
C
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide In 1,4-dioxane; water at 37.7℃; relative rate constant; other o- and p-substituted phenyl phosphomium salts; other temperature; | A 96% B 96.5% C 3.5% |


| Conditions | Yield |
|---|---|
| With copper(l) iodide; 6,7-dihydro-5H-quinolin-8-one oxime; potassium hydroxide In water at 85℃; for 24h; Inert atmosphere; | 95% |
| With potassium hydroxide In N,N-dimethyl-formamide at 110℃; for 18h; Buchwald-Hartwig Coupling; | 73% |
| at 250 - 260℃; |

| Conditions | Yield |
|---|---|
| With potassium tert-butylate; N,N-dimethyl-formamide at 35℃; for 24h; Schlenk technique; Inert atmosphere; Irradiation; | 95% |
| With allyl-trimethyl-silane In cyclohexane Quantum yield; Further Variations:; Solvents; UV-irradiation; | |
| With [hmim][ClO4] for 16h; Irradiation; | 78 % Chromat. |
| In isopropyl alcohol for 4h; Irradiation; Inert atmosphere; | 99 %Chromat. |
| With triethanolamine; water In ethanol for 24h; Irradiation; Glovebox; | 90 %Chromat. |

| Conditions | Yield |
|---|---|
| With hydrogen In toluene at 140℃; under 30003 Torr; for 3h; chemoselective reaction; | 95% |
| With tetrakis(triphenylphosphine)platinum; phenylsilane; C30H51Cl3Mo3N6PPtS4(1+)*BF4(1-) In tetrahydrofuran at 70℃; under 760.051 Torr; Inert atmosphere; Schlenk technique; chemoselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| With N-methylpyrrolidine zinc borohydride; sulfuric acid In tetrahydrofuran; water at 0 - 10℃; | 94% |
| With sodium cyanoborohydride for 2h; | 92% |
| With acetic acid; zinc In 1,4-dioxane at 30℃; for 0.5h; | 92% |
-
-
23145-45-1
(benzyl)di(methyl)(phenyl)ammonium bromide

-
-
121-69-7
N,N-dimethyl-aniline

| Conditions | Yield |
|---|---|
| With sodium hydrogen telluride In N,N-dimethyl-formamide at 40℃; for 4h; | 94% |
| With sodium hydrogen telluride In N,N-dimethyl-formamide at 40℃; for 4h; Mechanism; other phase transfer catalysts; |

-
A
-
13453-37-7
thallium(III) iodide

-
B
-
2923-17-3
lithium trifluoroacetate

-
C
-
121-69-7
N,N-dimethyl-aniline

| Conditions | Yield |
|---|---|
| In acetone -70°C, several hours, warming to room temp.; redn. of vol. (distn., vac., room temp.), pptn. on addn. of water, crystn. (overnight), dissolving (acetone), pptn. on pentane addn., crystn. (several days); | A 94% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With [Ru(Triphos)(TMM)]; hydrogen; bis(trifluoromethanesulfonyl)amide In tetrahydrofuran at 150℃; under 60006 Torr; for 15h; Inert atmosphere; | 94% |
| With [RhCl{κ3-P,C,P′=C(NCH2PCy2)2C10H6}]; phenylsilane In toluene at 90℃; for 16h; Schlenk technique; | 93% |
| With hydrogen In hexane at 140℃; under 15001.5 - 60006 Torr; for 7h; Inert atmosphere; Autoclave; | 92% |
-
-
124-38-9
carbon dioxide

-
-
100-61-8
N-methylaniline

-
A
-
121-69-7
N,N-dimethyl-aniline

-
B
-
93-61-8
N-methyl-N-phenylformamide

| Conditions | Yield |
|---|---|
| With phenylsilane In N,N-dimethyl acetamide at 60℃; for 4h; Time; Sealed tube; | A 94% B 8% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile at 110℃; under 760.051 Torr; for 48h; Mechanism; Solvent; Reagent/catalyst; Schlenk technique; | A 92% B 8% |
| With phenylsilane; C23H23O2P In acetonitrile at 100℃; under 15001.5 Torr; for 24h; | A 8% B 91% |

-
-
28611-39-4
4-(dimethylamino)benzene-boronic acid

-
-
121-69-7
N,N-dimethyl-aniline

| Conditions | Yield |
|---|---|
| With water In dimethyl sulfoxide at 100℃; for 2h; Reagent/catalyst; Solvent; Enzymatic reaction; | 94% |
| Multi-step reaction with 2 steps 1: dimethyl sulfoxide / toluene / 0.5 h / 120 °C / Dean-Stark 2: water / dimethylsulfoxide-d6 / 168 h / 100 °C View Scheme | |
| Multi-step reaction with 2 steps 1: N,N-dimethyl-formamide / 1 h / 120 °C / Molecular sieve; Inert atmosphere 2: water / dimethylsulfoxide-d6 / 168 h / 100 °C View Scheme | |
| Multi-step reaction with 2 steps 1: toluene / 1 h / 20 °C / Inert atmosphere 2: water / dimethylsulfoxide-d6 / 96 h / 100 °C View Scheme |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In 2,2,2-trifluoroethanol for 1.1h; Reflux; | 93% |
| Stage #1: formaldehyd; N-methylaniline With hydrogenchloride In methanol at 20℃; Stage #2: With N-methylpiperidine zinc borohydride In methanol at 20℃; for 0.25h; | 88% |
| With N-methylpyrrolidine zinc borohydride; sulfuric acid In tetrahydrofuran; water at 0 - 10℃; for 0.333333h; | 88% |

| Conditions | Yield |
|---|---|
| With 4a-FlEt-OOH In 1,4-dioxane at 30℃; Rate constant; | 100% |
| With oxygen; ruthenium trichloride In 1,2-dichloro-ethane at 20℃; under 760 Torr; for 8h; | 98% |
| With dihydrogen peroxide In acetonitrile at 80℃; under 760.051 Torr; for 4h; | 98% |

| Conditions | Yield |
|---|---|
| With bis(trimethylsilyl)sulphate at 170℃; for 5h; | 100% |
| Stage #1: N,N-dimethyl-aniline With bis(trimethylsilyl)sulphate at 170℃; for 4h; Stage #2: With water | 96% |
| With bis(trimethylsilyl)sulphate at 170℃; for 4h; | 85% |

| Conditions | Yield |
|---|---|
| With bismuth(III) nitrate; sulfuric acid; silica gel at 25℃; for 0.0333333h; | 100% |
| With sodium nitrate In neat (no solvent) at 20℃; for 0.05h; Green chemistry; | 98% |
| With 2-chloro-1-methyl-pyridinium iodide; water; silica gel; sodium nitrite In hexane at 20℃; for 0.75h; regioselective reaction; | 85% |

| Conditions | Yield |
|---|---|
| In methanol for 2h; Ambient temperature; | 100% |
-
-
129075-92-9
(4-Benzotriazol-1-ylmethyl-phenyl)-diethyl-amine

-
-
121-69-7
N,N-dimethyl-aniline

-
-
129075-94-1
C19H26N2

| Conditions | Yield |
|---|---|
| In acetic acid for 3h; Heating; | 100% |
-
-
98-88-4
benzoyl chloride

-
-
121-69-7
N,N-dimethyl-aniline

-
-
4931-66-2, 54571-66-3, 64700-65-8
methyl 5-oxopyrrolidine-2-carboxylate

-
-
1934-92-5
N-methyl-N-phenyl-benzamide

| Conditions | Yield |
|---|---|
| Product distribution; | 100% |

-
-
121-69-7
N,N-dimethyl-aniline

-
-
515145-68-3
bis(2-amino-4-nitrophenyl) ether

| Conditions | Yield |
|---|---|
| Stage #1: bis(2-amino-4-nitrophenyl) ether With hydrogenchloride; sodium nitrite at 0 - 5℃; for 1h; Stage #2: N,N-dimethyl-aniline With hydrogenchloride In acetate buffer pH=5; | 100% |

| Conditions | Yield |
|---|---|
| In benzene at 23℃; for 48h; | 100% |

| Conditions | Yield |
|---|---|
| In toluene Product distribution / selectivity; | 100% |
| In neat (no solvent) addn. of diborane to amine at liquid N2 temp., keeping at -111°C, -78°C and 0°C consecutively, stirring at 0°C for 4-6 h (vacuum line); cooling to -78°C, removal of B2H6 by distn.; | |
| In neat (no solvent) at 0°C;; | |
| In neat (no solvent) at room temp.;; |
-
-
113860-02-9
[Cp*Ru(CH3CN)3]OTf

-
-
121-69-7
N,N-dimethyl-aniline

| Conditions | Yield |
|---|---|
| In tetrahydrofuran byproducts: CH3CN; under N2; addn. of Ru-complex to N,N-dimethylaniline and THF (benzene-free), mixt. stirred (30°C); addn. of hexane, solid filtered, washed twice (hexane), dried (vac.), elem. anal.; | 100% |
-
-
258519-28-7
[((CH3)3CC(NC(CH3)3)2)GaCH3](1+)*[B(C6F5)4](1-)=[((CH3)3CC(NC(CH3)3)2)GaCH3][B(C6F5)4]

-
-
121-69-7
N,N-dimethyl-aniline

-
-
258519-32-3
[((CH3)3CC(NC(CH3)3)2)Ga(CH3)(N(CH3)2C6H5)](1+)*[B(C6F5)4](1-)=[((CH3)3CC(NC(CH3)3)2)Ga(CH3)(N(CH3)2C6H5)][B(C6F5)4]

| Conditions | Yield |
|---|---|
| In further solvent(s) under N2; in C6D5Cl, 23°C, 10 min; not isolated; NMR; | 100% |

| Conditions | Yield |
|---|---|
| With C47H45Cl2N5Ru2(2+)*2F6P(1-); dihydrogen peroxide; acetic acid In methanol at 60℃; for 8h; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; | 100% |
| With dihydrogen peroxide; acetic acid In water at 20℃; for 0.416667h; Reagent/catalyst; Solvent; | 98% |
| With C22H26N2O5U; dihydrogen peroxide; acetic acid In methanol at 20℃; for 8h; Catalytic behavior; Reagent/catalyst; Solvent; Irradiation; | 97% |
-
-
1493-13-6
trifluorormethanesulfonic acid

-
-
121-69-7
N,N-dimethyl-aniline

-
-
142279-32-1
N,N-dimethylphenylammonium trifluoromethanesulfonate

| Conditions | Yield |
|---|---|
| In hexane at 20℃; for 1h; | 100% |
-
-
121-69-7
N,N-dimethyl-aniline

-
-
33454-16-9
3-iodo-N,N-dimethylaniline

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-aniline With (TMEDA)Na(TMP)(t-Bu)Zn(t-Bu) In hexane at 20℃; Inert atmosphere; Stage #2: With iodine Inert atmosphere; | 100% |
-
-
121-69-7
N,N-dimethyl-aniline

-
-
199620-14-9, 13007-92-6
chromium(0) hexacarbonyl

-
-
124916-48-9, 14122-95-3, 12109-10-3
(N,N-dimethylaniline)tricarbonylchromium

| Conditions | Yield |
|---|---|
| In decalin byproducts: CO; 3.5:1 mixture of arene and Cr(CO)6 in solvent purged for 20 min with Ar, evacuated for 20 min, mixture refluxed, cooled to 20°C, cooled to -18°C under argon, reaction time 5.5 h; filtered off, dissolved in benzene, filtered through Kieselguhr, concentrated, light petroleum added, crystn. at -18°C, complexes eluted with benzene/ethyl acetate, elem. anal., NMR; | 99.5% |
| In neat (no solvent) boiling;; | 91% |
| In neat (no solvent) boiling;; | 91% |

| Conditions | Yield |
|---|---|
| With aluminum (III) chloride; sodium pyrophosphate; hydrazine hydrate; pyrographite; ammonium hydroxide; 1-butyl-3-methylimidazolium chloride; sodium hydroxide at 130℃; for 0.0222222h; Reagent/catalyst; Temperature; | 99.4% |
| With acetic acid; β-naphthol In ethanol at 20℃; for 12h; Mannich type Friedel-Crafts reaction; | 90% |
| With acetic acid In water | 82% |
-
-
121-69-7
N,N-dimethyl-aniline

-
A
-
14503-46-9
2-dimethylamino-benzenesulfonic acid

-
B
-
121-58-4
p-N,N-dimethylaminobenzenesulfonic acid

| Conditions | Yield |
|---|---|
| With chlorosulfonic acid In 1,2-dichloro-benzene at 50℃; for 1h; Product distribution; Kinetics; Mechanism; E(activ), oth. temperatures; | A 0.6% B 99.4% |
| With sulfur trioxide In 1,2-dichloro-ethane at 5℃; Rate constant; Thermodynamic data; Product distribution; oth. temperature, E(activ.), var. ratios of reactants; | |
| With sulfuric acid In 1,2-dichloro-benzene at 180℃; Kinetics; Thermodynamic data; Equilibrium constant; variation of molar ratio and temperature, Ea, ΔGo, ΔHo, ΔSo; | |
| With sulfuric acid In 1,2-dichloro-benzene at 24.9℃; Thermodynamic data; Mechanism; Activation Free Energy, Enthalpy, Entropy of sulfonation and desulfonation; |
-
-
121-69-7
N,N-dimethyl-aniline

-
-
1588-83-6
4-amino-3-nitrobenzoic acid

-
-
392300-99-1
4'-dimethylamino-2-nitroazobenzene-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 4-amino-3-nitrobenzoic acid With hydrogenchloride; sodium nitrite In water; acetic acid at 15 - 20℃; for 0.25h; Stage #2: N,N-dimethyl-aniline In water; acetic acid at 0 - 5℃; | 99.4% |
-
-
670-54-2
ethenetetracarbonitrile

-
-
121-69-7
N,N-dimethyl-aniline

-
-
6673-15-0
2-(4-dimethylaminophenyl)ethylene-1,1,2-tricarbonitrile

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20 - 25℃; for 0.0833333h; Sonication; | 99% |
| In N,N-dimethyl-formamide at 50 - 60℃; for 0.5h; | 95% |
| With choline chloride; urea at 35℃; for 0.0833333h; Reagent/catalyst; Solvent; Green chemistry; | 89% |
| With N,N-dimethyl-formamide | |
| In N,N-dimethyl-formamide |
-
-
556-56-9
allyl iodid

-
-
121-69-7
N,N-dimethyl-aniline

-
-
73680-59-8
N-allyl-N,N-dimethylbenzenaminium iodide

| Conditions | Yield |
|---|---|
| at 30℃; | 99% |

| Conditions | Yield |
|---|---|
| With methanol; tetraethylammonium chloride; bromine In dichloromethane at 35℃; other substituted anilines: regioselectivity of bromination; | 99% |
| With methanol; tetraethylammonium chloride; bromine In dichloromethane at 35℃; | 99% |
| With hexabromocyclopenta-1,3-diene; triethylamine In acetonitrile for 24h; Ambient temperature; | 99% |

| Conditions | Yield |
|---|---|
| With iodine at 30℃; for 12h; Green chemistry; | 99% |
| With tetrafluoroboric acid; [bis(pyridine)iodine]+ tetrafluoroborate In diethyl ether; dichloromethane for 0.1h; Ambient temperature; | 98% |
| With iodine In 1,4-dioxane; pyridine at 0 - 20℃; | 98% |
-
-
121-69-7
N,N-dimethyl-aniline

-
-
35462-54-5
N,N-Dimethylaniline hydroiodide

| Conditions | Yield |
|---|---|
| With hydrogen iodide In 1,4-dioxane; water at 0 - 20℃; for 12h; | 99% |
| With hydrogen iodide |
N,N-Dimethylaniline Consensus Reports
N,N-Dimethylaniline Standards and Recommendations
ACGIH TLV: TWA 5 ppm; STEL 10 ppm (skin); Not Classifiable as a Human Carcinogen
DFG MAK: 5 ppm (25 mg/m3); Confirmed Animal Carcinogen with Unknown Relevance to Humans
DOT Classification: 6.1; Label: Poison
N,N-Dimethylaniline Specification
N,N-Dimethylaniline is an organic compound with the formula C8H11N, and its systematic name is the same with the product name. With the CAS registry number 121-69-7, it is also named as N,N-Dimethylaminobenzene. It belongs to the product categories of Intermediates of Dyes and Pigments; Anilines, Aromatic Amines and Nitro Compounds; Organics; C-D, Puriss p.a. ACSNitrogen Compounds; Amines; Analytical Reagents for General Use; C8; Puriss p.a. ACS; C8 Essential Chemicals; Nitrogen Compounds; Reagent Plus; Routine Reagents; Organic Chemical. Its EINECS number is 204-493-5. In addition, the molecular weight is 121.18. Its classification codes are: (1)Human Data; (2)Mutation data; (3)Skin / Eye Irritant; (4)TSCA Flag T [Subject to the Section 4 test rule under TSCA]; (5)Tumor data. This chemical should be sealed and stored in a cool and dry place. Moreover, it should be protected from moisture, heat and fire. This chemical is a key precursor to commercially important triarylmethane dyes such as Malachite green and Crystal violet. It serves as a promoter in the curing of polyester and vinyl ester resins. It is also used as a precursor to other organic compounds.
Physical properties of N,N-Dimethylaniline are:
(1)ACD/LogP: 2.135; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.99; (4)ACD/LogD (pH 7.4): 2.13; (5)ACD/BCF (pH 5.5): 17.70; (6)ACD/BCF (pH 7.4): 24.59; (7)ACD/KOC (pH 5.5): 247.57; (8)ACD/KOC (pH 7.4): 343.97; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 3.24 Å2; (13)Index of Refraction: 1.55; (14)Molar Refractivity: 40.566 cm3; (15)Molar Volume: 127.425 cm3; (16)Polarizability: 16.082×10-24cm3; (17)Surface Tension: 34.71 dyne/cm; (18)Density: 0.951 g/cm3; (19)Flash Point: 62.778 °C; (20)Enthalpy of Vaporization: 42.974 kJ/mol; (21)Boiling Point: 193.539 °C at 760 mmHg; (22)Vapour Pressure: 0.46 mmHg at 25°C.
Preparation of N,N-Dimethylaniline:
N,N-Dimethylaniline can be prepared by N-benzyl-N,N-dimethyl-anilinium; bromide at the temperature of 40 °C. This reaction will need reagent NaTeH and solvent dimethylformamide with the reaction time of 4 hours. The yield is about 94%.
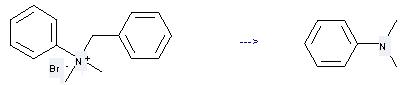
Uses of N,N-Dimethylaniline:
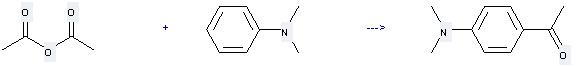
N,N-Dimethylaniline can be used to produce 1-(4-dimethylamino-phenyl)-ethanone at the temperature of 50 °C. It will need reagent Yb(OTf)3 and solvent nitromethane with the reaction time of 18 hours. The yield is about 76%.
Safety information of N,N-Dimethylaniline:
When you are using this chemical, please be cautious about it as the following:N,N-Dimethylaniline is harmful by inhalation and in contact with skin. It is toxic by inhalation, in contact with skin and if swallowed. It has a limited evidence of a carcinogenic effect. This substance is toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. After contact with skin, you should wash immediately with plenty of ... (to be specified by the manufacturer). When using it, you need to wear suitable protective clothing and gloves. In case of accident or if you feel unwell, you must seek medical advice immediately (show the label where possible). It should be avoided exposure, and you need to obtain special instructions before use. You must avoid releasing it to the environment, and you need to refer to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: N(c1ccccc1)(C)C
(2)Std. InChI: InChI=1S/C8H11N/c1-9(2)8-6-4-3-5-7-8/h3-7H,1-2H3
(3)Std. InChIKey: JLTDJTHDQAWBAV-UHFFFAOYSA-N
The toxicity data of N,N-Dimethylaniline is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | skin | > 20mL/kg (20mL/kg) | SKIN AND APPENDAGES (SKIN): "DERMATITIS, OTHER: AFTER SYSTEMIC EXPOSURE" | National Technical Information Service. Vol. OTS0571982, |
| human | LDLo | oral | 50mg/kg (50mg/kg) | GASTROINTESTINAL: NAUSEA OR VOMITING GASTROINTESTINAL: OTHER CHANGES | National Clearinghouse for Poison Control Centers, Bulletin. Vol. Jan/Feb, Pg. 1969, |
| mouse | LDLo | oral | 350mg/kg (350mg/kg) | National Toxicology Program Technical Report Series. Vol. NTP-TR-360, Pg. 1989, | |
| rabbit | LD50 | skin | 1770uL/kg (1.77mL/kg) | American Industrial Hygiene Association Journal. Vol. 23, Pg. 95, 1962. | |
| rat | LCLo | inhalation | 250mg/m3/4H (250mg/m3) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: EXCITEMENT | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 37(4), Pg. 35, 1972. |
| rat | LD50 | oral | 951mg/kg (951mg/kg) | BEHAVIORAL: TREMOR BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: CYANOSIS | National Technical Information Service. Vol. OTS0571982, |
| rat | LDLo | subcutaneous | 100mg/kg (100mg/kg) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 55, 1982. |
Related Products
- N-[(10-Oxido-9,10-dihydro-9-oxa-10-phosphaphenanthrene)methyl]-1,3,5-triazine-2,4,6-triamine
- N10-(Trifluoroacetyl)pteroic acid
- N-[1,1'-Biphenyl]-4-yl-9,9-dimethyl-9H-fluoren-2-amine
- N-[1,1'-Biphenyl]-4-yl-9,9-dimethyl-9H-fluoren-3-amine
- N-[1,1'-Biphenyl]-4-yl-N-(4-bromophenyl)-9,9-dimethyl-9H-fluoren-2-amine
- N-[1,1-Bis[(acetyloxy)methyl]-3-(4-octylphenyl)propyl]acetamide
- N'-[(1,1-Dimethylethoxy)carbonyl]-N-[(9H-fluoren-9-ylmethoxy)carbonyl]-N'-methyl-L-lysine
- N1,1-Diphenyl-1,2-ethanediamine
- N-(1,2-Dimethylpropyl)-2-pyridinamine
- N<sup xmlns="">1</sup>-(3,4-DIMETHYL-5-ISOXAZOLYL)SULFANIL-AMIDE LITHIUM SALT
- 121697-66-3
- 121700-26-3
- 121700-27-4
- 121706-11-4
- 121-71-1
- 121714-18-9
- 121714-22-5
- 121716-34-5
- 121716-35-6
- 121720-53-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View