-
Name
N-(2-Naphthyl)aniline
- EINECS 205-223-9
- CAS No. 135-88-6
- Article Data136
- CAS DataBase
- Density 1.156 g/cm3
- Solubility <0.1 g/100 mL at 19 °C in water
- Melting Point 105-108 °C(lit.)
- Formula C16H13N
- Boiling Point 395.5 °C at 760 mmHg
- Molecular Weight 219.286
- Flash Point 209.2 °C
- Transport Information
- Appearance light grey powder or crystals
- Safety 22-24/25-61-36/37-26
- Risk Codes 36/38-40-43-51/53-53-51
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, N
N
- Synonyms 2-Naphthylamine,N-phenyl- (7CI,8CI);2-Anilinonaphthalene;2-Naphthylphenylamine;Aceto PBN;AgeRite Powder;Antioxidant 116;Antioxidant D;Antioxidant PBN;N-(2-Naphthyl)aniline;N-Phenyl-b-naphthylamine;Naftam 2;Neozon D;Neozone;Neozone D;Phenyl-2-naphthylamine;N-Phenyl-2-naphthylamine;
- PSA 12.03000
- LogP 4.65640
Synthetic route
-
-
532-02-5
2-naphthalenesulfonic acid sodium salt

-
-
1865-45-8
sodium anilide

-
-
135-88-6
N-Phenyl-2-naphthylamine

| Conditions | Yield |
|---|---|
| With aniline at 290℃; for 0.25h; | 99% |
| With aniline at 290℃; for 0.25h; Product distribution; other temp.: 190 to 270 deg. C; other reaction time: 0.5 to 12 h; | 99% |

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; bis(dibenzylideneacetone)-palladium(0); sodium t-butanolate In toluene at 85℃; for 8h; | 99% |
-
-
42096-34-4
naphthalen-2-yl 1,1,2,2,3,3,4,4,4-nonafluorobutane-1-sulfonate

-
-
62-53-3
aniline

-
-
135-88-6
N-Phenyl-2-naphthylamine

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); 2,4,6-(i-Pr)3-[2-P(t-Bu)2-phenyl]benzene; 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 150℃; for 0.5h; microwave irradiation; | 99% |

| Conditions | Yield |
|---|---|
| With copper(II) acetate monohydrate; triethylamine In acetonitrile at 20℃; for 24h; Chan-Lam Coupling; | 99% |

| Conditions | Yield |
|---|---|
| With bis(η3-allyl-μ-chloropalladium(II)); potassium tert-butylate; 1,3-bis[(2,6-diisopropyl)phenyl]imidazolinium chloride In 1,4-dioxane at 100℃; for 1.5h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With bis[chloro(1,2,3-trihapto-allylbenzene)palladium(II)]; N-[2-(di(1-adamantyl)phosphino)phenyl]morpholine; potassium carbonate at 130℃; for 4h; Schlenk technique; Inert atmosphere; Sealed tube; | 98% |
| With N-[2-(di(1-adamantyl)phosphino)phenyl]morpholine; [Pd(π-cinnamyl)Cl]2 at 130℃; for 4h; Schlenk technique; Inert atmosphere; Sealed tube; | 98% |
| With NiCl(1-naphthyl)(PPh3)2; sodium hydride; 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride In 1,4-dioxane at 110℃; Inert atmosphere; | 75% |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); lithium hexamethyldisilazane; 1,2-bis-(dicyclohexylphosphino)ethane In toluene at 100℃; for 12h; Buchwald-Hartwig Coupling; | 97% |

| Conditions | Yield |
|---|---|
| With di-tert-butyl{2′-isopropoxy-[1,1′-binaphthalen]-2-yl}phosphane; potassium phosphate; palladium diacetate; phenylboronic acid In butan-1-ol at 110℃; for 15h; Inert atmosphere; | 96% |
| With (1,3-bis(2,6-diisopropylphenyl)imidazolidene)Ni(styrene)2; lithium tert-butoxide In 1,4-dioxane for 10h; Inert atmosphere; Heating; Schlenk technique; | 93% |
| With sodium t-butanolate; 1,3-bis[2,6-diisopropylphenyl]imidazolium chloride; chlorobis(triphenylphosphine)(1-naphthyl)nickel(II) In 1,4-dioxane at 110℃; for 0.5h; | 92% |

| Conditions | Yield |
|---|---|
| With aniline In water | 94% |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; sodium t-butanolate; 1,3-diisopropyl-1H-imidazol-3-ium chloride In 1,2-dimethoxyethane at 80℃; for 12h; Inert atmosphere; | 94% |
| With [Cu(N,N'-bis(diisopropylphosphino)-N,N'-dimethyl-2,6-diaminopyridine)Br]; potassium tert-butylate In tetrahydrofuran at 110℃; for 16h; Inert atmosphere; Sealed tube; | 86% |
| With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate In toluene at 40℃; | 82% |
| With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate In toluene at 40℃; | 82% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In 1-methyl-pyrrolidin-2-one at 150℃; for 24h; Inert atmosphere; Sealed vessel; | 94% |

| Conditions | Yield |
|---|---|
| With ethene; 5%-palladium/activated carbon In 5,5-dimethyl-1,3-cyclohexadiene at 150℃; under 760.051 Torr; for 24h; Molecular sieve; Autoclave; | 94% |
| With sulfur; oxygen In pyridine; cyclohexane at 110℃; for 24h; Sealed tube; | 63% |
| With gold-palladium bimetallic nanoparticles supported on TiO2 In 1,3,5-trimethyl-benzene at 160℃; under 760.051 Torr; for 24h; Inert atmosphere; Schlenk technique; | 66 %Chromat. |
-
-
74495-79-7
N-(2-naphthyl)-N-phenyl-acetamide

-
-
135-88-6
N-Phenyl-2-naphthylamine

| Conditions | Yield |
|---|---|
| With triethyl borane; sodium hydroxide In tert-butyl methyl ether at 80℃; for 6h; Inert atmosphere; Sealed tube; | 92% |
| Stage #1: N-(2-naphthyl)-N-phenyl-acetamide With Triethoxysilane; sodium triethylborohydride In tert-butyl methyl ether at 80℃; for 6h; Stage #2: With hydrogenchloride In tert-butyl methyl ether; water at 20℃; for 1h; chemoselective reaction; | 89% |
| Multi-step reaction with 2 steps 1: potassium hydroxide; triethyl borane / tetrahydrofuran / 24 h / 25 °C / Inert atmosphere; Schlenk technique; Sealed tube 2: sodium hydroxide; water / tetrahydrofuran / 1 h / 25 °C / Inert atmosphere; Schlenk technique; Sealed tube View Scheme |
-
-
532-02-5
2-naphthalenesulfonic acid sodium salt

-
-
62-53-3
aniline

-
A
-
135-88-6
N-Phenyl-2-naphthylamine

-
B
-
135-19-3
β-naphthol

| Conditions | Yield |
|---|---|
| With potassium hydroxide at 230℃; for 6h; Product distribution; other bases: NaOH + K2CO3, NaOH + Na2CO3, NaOH + KCl, NaOH + KF, NaOH; other temp.: 230 to 310 deg. C, other reaction time: 2 to 12 h; | A 90% B 3% |
| With sodium hydroxide; potassium carbonate at 310℃; for 4h; | A 34% B 65% |


| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate In toluene at 110℃; for 5.5h; Inert atmosphere; | 90% |
| With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate In toluene at 80℃; Buchwald-Hartwig Coupling; | 83% |
| With tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine; sodium t-butanolate In toluene at 80℃; | 80% |

| Conditions | Yield |
|---|---|
| With PdCl2(N,N’-bis-(2,6-di(iso-propyl)phenyl)imidazolidin-2-ylidene)(AsPh3); potassium tert-butylate In 1,4-dioxane at 110℃; for 4h; Buchwald-Hartwig Coupling; | 88% |

| Conditions | Yield |
|---|---|
| With copper diacetate; triethylamine In dichloromethane at 0 - 5℃; for 4h; Sonication; Green chemistry; | 87% |
| With N-(pyrid-2-yl)benzamide; nickel(II) acetate tetrahydrate; N,N,N',N'-tetramethylguanidine In toluene at 60℃; for 24h; Chan-Lam Coupling; | 80% |
| With [Cu(4-(dimethylamino)pyridine)4I]I In methanol at 20℃; for 0.25h; Chan-Lam Coupling; | 70% |

| Conditions | Yield |
|---|---|
| Stage #1: β-naphthol With potassium hydroxide In water; toluene at 20℃; Flow reactor; Stage #2: aniline With methanesulfonato(2-dicyclohexylphosphino-3,6-dimethoxy-2',4',6'-tri-i-propyl-1,1'-biphenyl)(2'-methylamino-1,1'-biphenyl-2-yl)palladium(II) In water; toluene at 120℃; Flow reactor; | 85% |
| With p-toluene sulfonic acid - choline chloride covalently immobilized on polymeric microspheres coated iron oxide magnetic nanoparticles In neat (no solvent) at 120℃; for 12h; chemoselective reaction; | 81% |
| With boron trifluoride diethyl etherate at 80℃; for 15h; Inert atmosphere; | 77% |
-
-
108-90-7
chlorobenzene

-
-
91-59-8
naphthalen-2-ylamine

-
A
-
135-88-6
N-Phenyl-2-naphthylamine

-
B
-
29601-75-0
1-phenyl-2-aminonaphthalene

| Conditions | Yield |
|---|---|
| With 2,2,6,6-tetramethylpiperidinyl-lithium In diethyl ether; cyclohexane at 25℃; for 24h; Temperature; Solvent; Reagent/catalyst; Inert atmosphere; | A n/a B 84% |


| Conditions | Yield |
|---|---|
| With copper(I) thiophene-2-carboxylate; sodium t-butanolate In dimethyl sulfoxide at 100℃; for 6h; Inert atmosphere; | 83% |
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; sodium t-butanolate In toluene at 120℃; Inert atmosphere; | 43% |
-
-
62-53-3
aniline

-
-
256652-04-7
4,4,5,5-tetramethyl-2-(naphthalen-2-yl)-1,3,2-dioxaborolane

-
-
135-88-6
N-Phenyl-2-naphthylamine

| Conditions | Yield |
|---|---|
| With copper diacetate; triethylamine In methanol; acetonitrile at 80℃; for 24h; Chan-Lam Coupling; Molecular sieve; Sealed tube; | 77% |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid In neat (no solvent) at 120℃; for 48h; Inert atmosphere; Sealed tube; | 75% |

| Conditions | Yield |
|---|---|
| Stage #1: nitrobenzene With [2,2]bipyridinyl; [MoO2Cl2(dmf)2] In toluene for 0.0333333h; Stage #2: naphthalene-2-boronic acid With triphenylphosphine In toluene at 100℃; for 20h; | 74% |

| Conditions | Yield |
|---|---|
| With indium; copper diacetate In methanol at 70℃; for 3h; | 70% |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); 1,3-bis-(2,6-diisopropylphenyl)-imidazol-2-ylidene In tetrahydrofuran; 1,4-dioxane at 150℃; for 24h; Kumada Cross-Coupling; Inert atmosphere; | 70% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; iodine; dimethyl sulfoxide In N,N,N,N,N,N-hexamethylphosphoric triamide; water at 110℃; for 20h; Schlenk technique; Sealed tube; Green chemistry; | 69% |
-
-
65739-06-2
3-[(4-methylphenyl)sulfonyl]-1-phenyltriaz-1-ene

-
-
32316-92-0
naphthalene-2-boronic acid

-
-
135-88-6
N-Phenyl-2-naphthylamine

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In toluene at 110℃; for 24h; Molecular sieve; Inert atmosphere; Schlenk technique; | 65% |
-
-
100-63-0
phenylhydrazine

-
-
91-59-8
naphthalen-2-ylamine

-
A
-
135-88-6
N-Phenyl-2-naphthylamine

-
B
-
1410783-23-1
N,1-diphenylnaphthalen-2-amine

| Conditions | Yield |
|---|---|
| With tetrabenzoporphyrinatocobalt(II); copper diacetate In acetonitrile at 0℃; for 13h; chemoselective reaction; | A 57% B 25% |

-
-
27067-31-8
N-phenyl 2-naphthyl nitroxide

-
A
-
6628-97-3
2-(phenylamino)naphthoquinone

-
B
-
21720-68-3
2-(phenylamino)-4-(phenylimino)-1(4H)-naphthalenone

-
C
-
135-88-6
N-Phenyl-2-naphthylamine

-
D
-
17704-02-8
N,N'-diphenyl-(1,1'-binaphthyl)-2,2'-diamine

| Conditions | Yield |
|---|---|
| In benzene for 336h; Ambient temperature; in the dark; Further byproducts given; | A 47% B 2.5% C 40% D 0.5% |


| Conditions | Yield |
|---|---|
| With sulfuric acid; silica gel; sodium nitrite In dichloromethane at 20℃; for 0.25h; | 99% |
| With trichloromelamine; silica gel; sodium nitrite In dichloromethane; water at 20℃; for 0.416667h; | 92% |
| With Nafion-H(R); silica gel; sodium nitrite In dichloromethane at 20℃; for 0.5h; | 75% |
-
-
135-88-6
N-Phenyl-2-naphthylamine

-
-
74-88-4
methyl iodide

-
-
6364-05-2
N-methyl-N-phenylnaphthalen-2-amine

| Conditions | Yield |
|---|---|
| Stage #1: N-Phenyl-2-naphthylamine With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 0.5h; Inert atmosphere; Stage #2: methyl iodide In tetrahydrofuran; hexane at -78 - 20℃; | 99% |

| Conditions | Yield |
|---|---|
| With tri-tert-butyl phosphine; sodium t-butanolate; palladium diacetate In toluene at 100℃; | 98% |
-
-
1273-86-5
1-ferrocenylmethanol

-
-
135-88-6
N-Phenyl-2-naphthylamine

-
-
93122-40-8
C5H5FeC5H4CH2N(C6H5)(C10H7)

| Conditions | Yield |
|---|---|
| With HClO4 or HBF4 In dichloromethane byproducts: H2O; aq. HClO4 (70 %) or HBF4 (45 %) added (vigorous stirring) to the Fe-complex and the amine; soln. stirred (30 min, room temp.), addn. of ether; ethereal soln. washed twice (water), dried (sodium sulfate), solvent removed (vac.), recrystn. (ethyl acetate); elem. anal.; | 98% |
-
-
38303-35-4
1,8-dibromopyrene

-
-
135-88-6
N-Phenyl-2-naphthylamine

| Conditions | Yield |
|---|---|
| With tri-tert-butyl phosphine; palladium diacetate; sodium t-butanolate In o-xylene at 125 - 130℃; for 3.5h; Buchwald-Hartwig Coupling; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With tri-tert-butyl phosphine; palladium diacetate; caesium carbonate In toluene at 110℃; for 20h; Inert atmosphere; | 96% |
-
-
135-88-6
N-Phenyl-2-naphthylamine

-
-
33816-55-6
N-phenyl-1,2,3,4-tetrahydronaphthalen-2-amine

| Conditions | Yield |
|---|---|
| With tris(pentafluorophenyl)borate; hydrogen In toluene at 60℃; under 15001.5 Torr; for 6h; Pressure; Solvent; Temperature; Inert atmosphere; Autoclave; | 94% |
| With nickel at 100 - 150℃; under 147102 Torr; Hydrogenation; | |
| With copper oxide-chromium oxide at 250 - 300℃; under 147102 Torr; Hydrogenation; |
-
-
90-11-9
1-Bromonaphthalene

-
-
135-88-6
N-Phenyl-2-naphthylamine

-
-
1400899-20-8
N-(naphthalen-2-yl)-N-phenylnaphthalen-1-amine

| Conditions | Yield |
|---|---|
| Stage #1: 1-Bromonaphthalene; N-Phenyl-2-naphthylamine With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; palladium diacetate In toluene Buchwald-Hartwig Coupling; Inert atmosphere; Sealed tube; Stage #2: With n-hexyllithium In hexane; toluene at 105℃; for 2h; Buchwald-Hartwig Coupling; Inert atmosphere; Sealed tube; | 94% |
| With 2-dicyclohexylphosphino-2',6'-dimethoxybiphenyl; n-hexyllithium; palladium diacetate In hexane; toluene at 105℃; for 2h; Buchwald-Hartwig Coupling; Inert atmosphere; | 94% |
-
-
135-88-6
N-Phenyl-2-naphthylamine

| Conditions | Yield |
|---|---|
| With dicyclohexyl-(2',6'-dimethoxybiphenyl-2-yl)-phosphane; tris-(dibenzylideneacetone)dipalladium(0); sodium t-butanolate In toluene at 100℃; for 5h; Buchwald-Hartwig Coupling; Inert atmosphere; | 94% |
-
-
135-88-6
N-Phenyl-2-naphthylamine

-
-
1467758-59-3
C13H13BrN2O3

| Conditions | Yield |
|---|---|
| With (R)-3,3'-bis(9-anthracenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate In dichloromethane at -60℃; for 35h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 94% |
-
-
1277-49-2
1-ferrocenylethanol

-
-
135-88-6
N-Phenyl-2-naphthylamine

-
-
93122-45-3
C5H5FeC5H4CH(CH3)N(C6H5)(C10H7)

| Conditions | Yield |
|---|---|
| With HClO4 or HBF4 In dichloromethane byproducts: H2O; aq. HClO4 (70 %) or HBF4 (45 %) added (vigorous stirring) to the Fe-complex and the amine; soln. stirred (30 min, room temp.), addn. of ether; ether soln. washed twice (water), dried (sodium sulfate), solvent removed (vac.); elem. anal.; | 93% |

-
-
135-88-6
N-Phenyl-2-naphthylamine

-
-
93122-48-6
C5H5FeC5H4CH(C6H5)N(C6H5)(C10H7)

| Conditions | Yield |
|---|---|
| With HBF4 or HClO4 In dichloromethane byproducts: H2O; aq. HBF4 (45 %) or HClO4 (70 %) added (vigorous stirring) to the Fe-complex and the amine; soln. stirred (30 min, room temp.), addn. of ether; ethereal soln. washed twice (water), dried (sodium sulfate), solvent removed (vac.), recrystn. (heptane); elem. anal.; | 91% |
-
-
135-88-6
N-Phenyl-2-naphthylamine

-
-
1373942-82-5
tert-butyl 2-oxoindolin-3-ylidenecarbamate

| Conditions | Yield |
|---|---|
| With (R)-3,3'-bis(9-anthracenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate In dichloromethane at -60℃; for 24h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 91% |

| Conditions | Yield |
|---|---|
| With sodium t-butanolate; tris-(dibenzylideneacetone)dipalladium(0); tri-tert-butyl phosphine In toluene at 90℃; for 3h; | 90% |
N-Phenyl-2-naphthylamine Consensus Reports
IARC Cancer Review: Group 3 IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 7 ,1987,p. 318.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 16 ,1978,p. 325.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 16 ,1978,p. 325.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Reported in EPA TSCA Inventory.
N-Phenyl-2-naphthylamine Standards and Recommendations
ACGIH TLV: Not Classifiable as a Human Carcinogen
DFG MAK: Confirmed Animal Carcinogen with Unknown Relevance to Humans
N-Phenyl-2-naphthylamine Specification
The N-Phenyl-beta-naphthylamine is an organic compound with the formula C16H13N. The IUPAC name of this chemical is N-phenylnaphthalen-2-amine. With the CAS registry number 135-88-6 and EINECS 205-223-9, it is also named as 2-Naphthylamine, N-phenyl-. The product's category is Industrial / Fine Chemicals. It is light grey powder or crystal which is insoluble in water, soluble in alcohol, carbon tetrachloride, benzene and acetone. Moverover, this chemical may gradually change into deep red when exposed to air or sunlight. Additionally, it should be stored in the cool and dry place.
Physical properties about N-Phenyl-beta-naphthylamine are: (1)ACD/LogP: 4.54; (2)ACD/LogD (pH 5.5): 4.54; (3)ACD/LogD (pH 7.4): 4.54; (4)ACD/BCF (pH 5.5): 1662.33; (5)ACD/BCF (pH 7.4): 1662.36; (6)ACD/KOC (pH 5.5): 7030.47; (7)ACD/KOC (pH 7.4): 7030.61; (8)#H bond acceptors: 1; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 2; (11)Index of Refraction: 1.702; (12)Molar Refractivity: 73.47 cm3; (13)Molar Volume: 189.59 cm3; (14)Polarizability: 29.126 10-24cm3; (15)Surface Tension: 50.1209983825684 dyne/cm; (16)Density: 1.157 g/cm3; (17)Flash Point: 209.226 °C; (18)Enthalpy of Vaporization: 64.567 kJ/mol; (19)Boiling Point: 395.498 °C at 760 mmHg
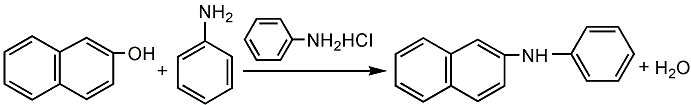
Preparation of N-Phenyl-beta-naphthylamine: It can be obtained by condensation of 2-naphthol and aniline in the presence of aniline hydrochloride at 250 °C. Add 2-naphthol and aniline in melting pot for fusion and add aniline hydrochloride for condensation. After the reaction finishing, add sodium carbonate for neutralization. Then steam out the excess aniline. Finally, after dehydration and dried under vacuum, we can get the product.
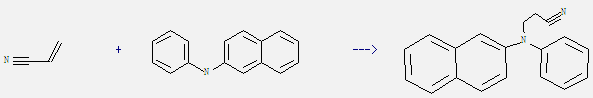
Uses of N-Phenyl-beta-naphthylamine: It is used for natural rubber, synthetic rubber and latex. And this material is universal antioxidant in natural rubber, diene synthetic rubber, chloroprene rubber and latex. What's more, it can react with acrylonitrile to get N-(b-naphthyl)-N-(phenyl)-b-aminopropionitrile. This reaction needs reagent gl. acetic acid. The yield is 85%.
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes and skin. And it has limited evidence of a carcinogenic effect. What;s more, it is toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. So people should not breathe dust and avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. If you want to contact this product, you must wear suitable protective clothing and gloves. Avoid release to the environment. Refer to special instructions / safety data sheets.
You can still convert the following datas into molecular structure:
1. SMILES:c3c(Nc1ccccc1)cc2ccccc2c3
2. InChI:InChI=1/C16H13N/c1-2-8-15(9-3-1)17-16-11-10-13-6-4-5-7-14(13)12-16/h1-12,17H
3. InChIKey:KEQFTVQCIQJIQW-UHFFFAOYAL
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 1450mg/kg (1450mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Hygiene and Sanitation Vol. 31(1-3), Pg. 183, 1966. |
| rat | LD50 | oral | 8730mg/kg (8730mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Hygiene and Sanitation Vol. 31(1-3), Pg. 183, 1966. |
| rat | LD50 | unreported | 8700mg/kg (8700mg/kg) | LIVER: OTHER CHANGES BRAIN AND COVERINGS: OTHER DEGENERATIVE CHANGES LUNGS, THORAX, OR RESPIRATION: ACUTE PULMONARY EDEMA | Tijdschrift Voor Sociale Geneeskunde. Vol. 53, Pg. 415, 1975. |
Related Products
- N-Phenyl-2-naphthylamine
- 135887-26-2
- 135889-00-8
- 13589-02-1
- 13589-72-5
- 135900-33-3
- 13590-42-6
- 135906-03-5
- 13590-82-4
- 135908-33-7
- 135908-43-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View