-
Name
Paclitaxel
- EINECS 205-285-7
- CAS No. 33069-62-4
- Article Data81
- CAS DataBase
- Density 1.39 g/cm3
- Solubility solubile in methanol: 50 mg/mL, clear, colorless
- Melting Point 213 °C
- Formula C47H51NO14
- Boiling Point 957.115 °C at 760 mmHg
- Molecular Weight 853.92
- Flash Point 532.644 °C
- Transport Information
- Appearance white powder
- Safety 22-26-36/37/39-45
- Risk Codes 37/38-41-42/43-62-68-40-48-20/21/22
-
Molecular Structure
- Hazard Symbols R40:; R41:;
- Synonyms BMS 181339-01;Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2a-alpha,4-beta,4a-beta,6-beta,9-alpha(alpha-R*,beta-S*),11-alpha,12-alpha,12a-alpha, 12b-alpha))-;Taxol.RTM. (Registered Trademark);QW 8184;MBT 0206;12-benzoate, 9-ester with (2R,3S)-N-benzoyl-3-phenylisoserine;Palcitaxel;Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-;Prestwick_459;Abraxane;Taxol (TN);Paxceed;NSC-125973;Paxene;Plaxicel;Onxol;Indirubin;Paclitaxle;Paclitaxel Semi-Synthetic USP30;
- PSA 221.29000
- LogP 4.12660
Synthetic route
-
-
135365-62-7
1-hydroxy-7β-triethylsilyloxy-9-oxo-10β-acetyloxy-5β,20-epoxytax-11-ene-2α,4,13α-triyl 4-acetate 2-benzoate 13-[(2R,3S)-3-benzoylamino-2-triethylsilyloxy-3-phenylpropanoate]

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With pyridine; hydrogen fluoride In water; acetonitrile at 0 - 25℃; for 14h; | 98% |
| With pyridine; hydrogen fluoride In water; acetonitrile at 0 - 25℃; for 14h; | 98% |
| With hydrogenchloride In methanol; water at -5 - 30℃; for 27h; | 90% |
| With pyridine hydrogenfluoride In tetrahydrofuran at 25℃; for 1.25h; | 80% |

| Conditions | Yield |
|---|---|
| Stage #1: With dmap; chloro-trimethyl-silane; diisopropylamine In tetrahydrofuran at 20℃; for 1h; Stage #2: Schwartz's reagent In tetrahydrofuran at 0℃; for 4h; Stage #3: benzoyl chloride With tert-butyl methyl ether; N,N-bis-(2-hydroxyethyl)glycine; sulfuric acid; sodium hydrogencarbonate more than 3 stages; | 86.6% |

| Conditions | Yield |
|---|---|
| With tert-butyl methyl ether; N,N-bis-(2-hydroxyethyl)glycine; sulfuric acid; pyridinium p-toluenesulfonate; sodium hydrogencarbonate; triethylamine; ethyl vinyl ether; Schwartz's reagent In tetrahydrofuran; water; ethyl acetate at 5 - 20℃; for 24.8333h; | 80% |

| Conditions | Yield |
|---|---|
| Stage #1: With dmap; chloro-trimethyl-silane; diisopropylamine In tetrahydrofuran at 0℃; for 1h; Stage #2: Schwartz's reagent In tetrahydrofuran at 5℃; for 4h; Stage #3: benzoyl chloride With tert-butyl methyl ether; N,N-bis-(2-hydroxyethyl)glycine; sulfuric acid; sodium chloride more than 3 stages; | 76.5% |
-
-
439813-57-7
(2'R,3'S)-2'-ethoxyethyl-7-triethylsilyl taxol

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol; water at 0℃; for 30h; | 90% |
| With hydrogenchloride In ethanol at 0℃; for 72h; | 80% |

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With pyridine; hydrogen fluoride In water; acetonitrile at 0 - 25℃; for 18h; | 99% |
| Stage #1: (2'R,3"S)-2'-(2-methoxy-2-propyloxy)-7-triethylsilyl taxol With trifluoroacetic acid In water; acetic acid at 20℃; for 7h; Stage #2: With sodium acetate In dichloromethane; water; acetic acid for 0.25h; | 26.3 g |

| Conditions | Yield |
|---|---|
| With tert-butyl methyl ether; N,N-bis-(2-hydroxyethyl)glycine; sulfuric acid; pyridinium p-toluenesulfonate; sodium hydrogencarbonate; triethylamine; ethyl vinyl ether; Schwartz's reagent In tetrahydrofuran; water; ethyl acetate at -5 - 20℃; for 26.8333h; | 43.6% |
| Stage #1: Schwartz's reagent In tetrahydrofuran at 5℃; for 4h; Stage #2: With N,N-bis-(2-hydroxyethyl)glycine In tetrahydrofuran; ethyl acetate for 0.833333h; Stage #3: benzoyl chloride With tert-butyl methyl ether; sulfuric acid; sodium hydrogencarbonate more than 3 stages; |
-
-
158722-23-7
7-(triethylsilyl)-13-O-[((4S,5R)-2,4-diphenyl-4,5-dihydrooxazol-5-yl)carbonyl]baccatin

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 60 - 80℃; for 3.5h; | 80% |
| With hydrogenchloride In methanol | 76% |
| With hydrogenchloride at 95℃; for 2h; | 75% |
| With hydrogenchloride In methanol at 60℃; for 3h; Hydrolysis; |
-
-
1122-58-3
dmap

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In ethanol at 0 - 20℃; for 2.41667h; | 100% |

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; | 93.3% |
-
-
162081-12-1
7-O-(2-chloroacetyl)paclitaxel

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With ammonia In pyridine at 0 - 5℃; for 12h; | 84% |
-
-
906370-55-6
13-O-[[(4S,5R)-3-N-benzoyl-4-phenyloxazolidin-2RS-methoxy-5-yl]carbonyl]-7-O-triethylsilylbaccatin III

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In ethanol; ethyl acetate at 45℃; for 4h; | 80% |

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With acetic acid; trifluoroacetic acid In water at 30℃; Temperature; Concentration; | 81% |
-
-
148930-55-6
7-O-(triethylsilyl)paclitaxel

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With pyridine hydrogenfluoride In tetrahydrofuran Ambient temperature; | 100% |
| With hydrogenchloride; water In ethanol at 20℃; for 2h; Hydrolysis; | 100% |
| With hydrogenchloride In ethanol; water at 0 - 20℃; for 2.41667h; | 100% |
| Multi-step reaction with 9 steps 1: benzoyl peroxide 2: HCl / acetonitrile 3: N-iodosuccinimide, 4 Angstroem sieves / CH2Cl2; tetrahydrofuran 4: H2 / Pd-C / ethyl acetate / 2.5 h / 3102.9 Torr / Ambient temperature 5: bovine intestinal alkaline phosphatase 6: benzoyl peroxide / acetonitrile / 2 h / 0 °C 7: N-iodosuccinimide, 4 Angstroem sieves / CH2Cl2; tetrahydrofuran 8: H2 / Pd-C / ethyl acetate / 2.5 h / 3102.9 Torr / Ambient temperature 9: bovine intestinal alkaline phosphatase, or incubation in mouse plasma at 37 deg C View Scheme | |
| Multi-step reaction with 5 steps 1: benzoyl peroxide 2: HCl / acetonitrile 3: N-iodosuccinimide, 4 Angstroem sieves / CH2Cl2; tetrahydrofuran 4: H2 / Pd-C / ethyl acetate / 2.5 h / 3102.9 Torr / Ambient temperature 5: bovine intestinal alkaline phosphatase View Scheme |

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 0℃; for 1.5h; Inert atmosphere; | A 67% B 88 mg |
-
-
1033962-72-9
13-[(2'R,3'S)-3'-benzoylamino-3'-phenyl-2'-hydroxypropinonyl]-7-trichloroacetylbaccatin III

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With methanol; ammonium acetate In tetrahydrofuran for 4h; | 80% |
| With ammonium acetate In tetrahydrofuran; methanol for 4h; | 80% |
-
-
307923-51-9
2'-O-benzoyl-3'-N-debenzoyl-paclitaxel

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With anthranilic acid; triethylamine In tetrahydrofuran; ethyl acetate | 65% |
| In dimethyl sulfoxide at 20℃; |

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 5h; | 91.8% |

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With tetrabutyl ammonium fluoride In tetrahydrofuran for 5h; | 91.5% |
-
-
718627-80-6
C62H73NO16Si

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran; methanol; water for 0.666667h; | 82% |

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0 - 20℃; for 12h; Inert atmosphere; | A 29% B 80 mg |

-
-
219780-99-1
2’-TES-7-TROC-taxol

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With acetic acid; zinc | 84% |
| With acetic acid; zinc at 20℃; for 16h; deacylation; | 84% |
-
-
133524-70-6
13-(O-2R-hydroxy-3S-amine-phenylpropionyl)-baccatin III

-
-
98-88-4
benzoyl chloride

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; potassium dihydrogenphosphate; sodium chloride In tetrahydrofuran; water at 20℃; for 0.25 - 0.5h; | 70% |
| With triethylamine In acetonitrile at 20℃; for 0.5h; Purification / work up; | |
| With triethylamine In acetonitrile at 20℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| Stage #1: 10-deacetylpaclitaxel With Chloroacetamide In tetrahydrofuran at 100℃; for 3h; Stage #2: acetic anhydride In tetrahydrofuran at 100℃; for 3h; Stage #3: With methanol; sodium hydrogencarbonate; thiourea at 20℃; for 1h; Product distribution / selectivity; | 78.8% |
| Stage #1: 10-deacetylpaclitaxel With pyridine; Chloroacetamide at 20℃; for 1h; Stage #2: acetic anhydride With pyridine In tetrahydrofuran at 100℃; for 0.5h; Stage #3: With methanol; sodium hydrogencarbonate; thiourea at 20℃; for 1h; Product distribution / selectivity; | 60% |
| Stage #1: 10-deacetylpaclitaxel With Chloroacetamide In tetrahydrofuran at 100℃; for 3h; Stage #2: acetic anhydride In tetrahydrofuran at 100℃; for 3h; Stage #3: With sodium hydrogencarbonate; thiourea In methanol at 20℃; for 1h; Product distribution / selectivity; |
-
-
115437-19-9
C57H73NO15Si

-
-
33069-62-4
taxol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol at 0℃; for 30h; | 89% |
Paclitaxel Chemical Properties
Systematic Name: (2alpha,5beta,7beta,10beta,13alpha)-4,10-bis(acetyloxy)-13-{[(2R,3S)-3-(benzoylamino)-2-hydroxy-3-phenylpropanoyl]oxy}-1,7-dihydroxy-9-oxo-5,20-epoxytax-11-en-2-yl benzoate
Synonyms of Paclitaxel (CAS NO.33069-62-4): 7,11-Methano-5H-cyclodeca[3,4]benz[1,2-b]oxete benzenepropanoic acid deriv ; Taxal ; Taxol A
Molecular Formula: C47H51NO14
Molecular Weight: 853.92
Molecular Structure: 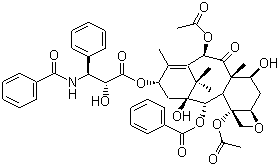
H bond acceptors: 15
H bond donors: 4
Freely Rotating Bonds: 17
Polar Surface Area: 221.29 Å2
Index of Refraction: 1.637
Molar Refractivity: 219.267 cm3
Molar Volume: 610.556 cm3
Surface Tension: 68.487 dyne/cm
Density: 1.399 g/cm3
Flash Point: 532.644 °C
Enthalpy of Vaporization: 145.984 kJ/mol
Boiling Point: 957.115 °C at 760 mmHg
Vapour Pressure: 0 mmHg at 25 °C
Melting Point : 213 °C
Appearance: White Powder
Solubility: solubile in methanol: 50 mg/mL, clear, colorless
Stability: Stable. Incompatible with strong oxidizing agents. Combustible.
Product Categories of Paclitaxel (CAS NO.33069-62-4): Active Pharmaceutical Ingredients; Pharmaceutical material and intermeidates; Antineoplastics;Antineoplastic; APIs; Antitumors for Research and Experimental Use; Biochemistry; Natural Plant Extract; Intermediates & Fine Chemicals; Pharmaceuticals; Natural Anti-cancer Medical Materials and It's Derivatives; Antitumour; Signalling
Paclitaxel Uses
Paclitaxel (CAS NO.33069-62-4) can be used to treat patients with breast cancer,lung, ovarian, head and neck cancer, and advanced forms of Kaposi's sarcoma. Besides, it is also can be used for the prevention of restenosis. And Paclitaxel also can be used in the study of structure and function of microtubles into tubulin.
Paclitaxel Production
From 1967 to 1993, Paclitaxel (CAS NO.33069-62-4) was derived from bark from the Pacific yew, the harvesting of which kills the tree in the process. From the late 1970s, chemists attempted a total synthesis of the molecule, starting from petrochemical-derived starting materials.
Paclitaxel Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | intravenous | 15mg/kg (15mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY STIMULATION | National Technical Information Service. Vol. PB83-170969, |
| mouse | LD50 | intraperitoneal | 128mg/kg (128mg/kg) | SKIN AND APPENDAGES (SKIN): HAIR: OTHER | National Technical Information Service. Vol. PB83-170969, |
| mouse | LD50 | intravenous | 12mg/kg (12mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Pharmaceutical Research. Vol. 4, Pg. 162, 1987. |
| rat | LD50 | intraperitoneal | 32530ug/kg (32.53mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | National Technical Information Service. Vol. PB83-170969, |
| rat | LDLo | intravenous | 85mg/kg (85mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BLOOD: CHANGES IN BONE MARROW NOT INCLUDED ABOVE | Journal of Toxicological Sciences. Vol. 19(Suppl, |
| women | LDLo | intravenous | 4995mg/kg/3H- (4995mg/kg) | CARDIAC: CARDIOMYOPATHY INCLUDING INFARCTION BLOOD: LEUKOPENIA BLOOD: THROMBOCYTOPENIA | Lancet. Vol. 343, Pg. 727, 1994. |
Paclitaxel Safety Profile
Safety Information about Paclitaxel (CAS NO.33069-62-4):
Hazard Codes:  Xn
Xn
Risk Statements: 37/38-41-42/43-62-68-40-48-20/21/22
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed.
R37/38: Irritating to respiratory system and skin.
R40: Limited evidence of a carcinogenic effect.
R41: Risk of serious damage to the eyes.
R42/43: May cause sensitization by inhalation and skin contact.
R48: Danger of serious damage to health by prolonged exposure.
R62: Risk of impaired fertility.
R68: Possible risk of irreversible effects.
Safety Statements: 22-26-36/37/39-45
S22: Do not breathe dust.
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
RIDADR: 1544
WGK Germany: 3
RTECS: DA8340700
F: 10-21
HazardClass: 6.1(b)
PackingGroup: III
Paclitaxel Specification
Paclitaxel (CAS NO.33069-62-4), its Synonyms are 7,11-Methano-5H-cyclodeca[3,4]benz[1,2-b]oxete benzenepropanoic acid deriv ; Taxal ; Taxol A .
Related Products
- Paclitaxel
- Paclitaxel side chain acid
- 3307-00-4
- 33070-31-4
- 33070-32-5
- 3307-37-7
- 3307-39-9
- 33075-00-2
- 330784-47-9
- 330785-81-4
- 330792-76-2
- 330793-01-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View