-
Name
Perindopril erbumine
- EINECS 600-798-6
- CAS No. 107133-36-8
- Article Data35
- CAS DataBase
- Density 1.15 g/cm3
- Solubility
- Melting Point 126-128 °C
- Formula C19H32N2O5.C4H11N
- Boiling Point 537.4 °C at 760 mmHg
- Molecular Weight 441.612
- Flash Point 278.8 °C
- Transport Information
- Appearance white solid
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms (2S,3aS,7aS)-1-((S)-N-((S)-1-Carboxybutyl)alanyl)hexahydro-2-indolinecarboxylic acid, 1-ethyl ester, compound with tert-butylamine (1:1);Aceon (TN);S 9490-3;Perindopril erbumine/tert-butylamine;Perindopril erbumine [USAN];(2S,3aS,7aS)-1-{(2S)-2-[(1S)-1-(ethoxycarbonyl)butylamino]propanoyl}octahydro-1H-indole-2-carboxylic acid--2-methylpropan-2-amine (1/1);(2S,3aS,7aS)-1-[(2S)-2-[[(1S)-1-ethoxycarbonylbutyl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole-2-carboxylic acid; 2-methylpropan-2-amine;S-9490-3;1H-Indole-2-carboxylic acid,1-[(2S)-2-[[(1S)-1-(ethoxycarbonyl)- butyl]amino]-1-oxopropyl]octahydro-,(2S,3aS,- 7aS)-,compd. with 2-methyl-2-propanamine (1:1);1H-Indole-2-carboxylic acid, 1-(2-((1-(ethoxycarbonyl)butyl)amino)-1-oxopropyl)octahydro-, (2S-(1(R*(R*)),2alpha,3abeta,7abeta))-, compd. with 2-methyl-2-propanamine (1:1);McN-A-2833-109;Perindopril erbumine (JAN/USAN);MCN-A 2833-109;Perindopril tert-butylamine;Perindopril;
- PSA 121.96000
- LogP 3.71330
Synthetic route

| Conditions | Yield |
|---|---|
| In ethyl acetate Heating / reflux; | 95% |
| In ethyl acetate Heating / reflux; | 95% |
| In ethyl acetate Heating / reflux; | 95% |
-
-
82834-16-0
Perindopril

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| With tert-butylamine In ethyl acetate; acetonitrile Heating / reflux; | 95% |
| With tert-butylamine In ethyl acetate at 20℃; for 1 - 1.5h; | 65% |
-
-
75-64-9
tert-butylamine

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Stage #1: (2S,3aS,7aS)-perhydroindole-2-carboxylic acid With tert-butylimino-tri(pyrrolidino)phosphorane In acetonitrile Stage #2: N-[1(S)-(ethoxycarbonyl)butyl]-L-alanine ester of acetonoxime In acetonitrile at 50℃; for 0.5h; Stage #3: tert-butylamine In dichloromethane at 0 - 10℃; for 0.5h; Product distribution / selectivity; | 90% |
| Stage #1: (2S,3aS,7aS)-perhydroindole-2-carboxylic acid With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile Stage #2: N-[1(S)-(ethoxycarbonyl)butyl]-L-alanine ester of acetonoxime In acetonitrile at 40 - 45℃; Stage #3: tert-butylamine With hydrogenchloride In acetonitrile at 50 - 60℃; Product distribution / selectivity; | 90% |
| Stage #1: benzylperindopril oxalate With hydrogen; 5%-palladium/activated carbon In ethanol at 30℃; under 3677.86 Torr; for 3h; Stage #2: tert-butylamine In ethanol Product distribution / selectivity; |

-
-
80875-98-5
(2S,3aS,7aS)-perhydroindole-2-carboxylic acid

-
-
75-64-9
tert-butylamine

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Stage #1: (2S,3aS,7aS)-perhydroindole-2-carboxylic acid With chloro-trimethyl-silane; triethylamine In dichloromethane at 20 - 25℃; for 2h; Stage #2: N-((S)-1-carbethoxybutyl)-L-alanyl chloride hydrochloride With triethylamine In dichloromethane at -15℃; for 2h; Stage #3: tert-butylamine In Isopropyl acetate Product distribution / selectivity; | 86% |
| Stage #1: (2S,3aS,7aS)-perhydroindole-2-carboxylic acid With chloro-trimethyl-silane; triethylamine In dichloromethane at 20 - 25℃; for 2h; Stage #2: N-((S)-1-carbethoxybutyl)-L-alanyl chloride hydrochloride With triethylamine In dichloromethane at -15℃; for 2h; Stage #3: tert-butylamine In ethyl acetate Product distribution / selectivity; | 80% |
| Stage #1: (2S,3aS,7aS)-perhydroindole-2-carboxylic acid With chloro-trimethyl-silane; triethylamine In dichloromethane at 20 - 25℃; for 2h; Stage #2: N-((S)-1-carbethoxybutyl)-L-alanyl chloride hydrochloride With triethylamine In dichloromethane at -15℃; for 2h; Stage #3: tert-butylamine In DMF (N,N-dimethyl-formamide) Product distribution / selectivity; | 78% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride; tert-butylamine; triethylamine In dichloromethane; water; acetonitrile | A 81% B n/a |

-
-
1159491-00-5
C12H23NO2Si

-
-
82834-12-6
N-<1(S)-(ethoxycarbonyl)butyl>-L-alanine

-
-
75-64-9
tert-butylamine

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Stage #1: N-<1(S)-(ethoxycarbonyl)butyl>-L-alanine With 1H-imidazole; thionyl chloride In dichloromethane at -10℃; for 2h; Stage #2: C12H23NO2Si In dichloromethane at -10 - 25℃; for 21h; Stage #3: tert-butylamine In acetonitrile Heating / reflux; | 61% |


| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethyl acetate under 2068.65 Torr; for 8h; |

| Conditions | Yield |
|---|---|
| In dichloromethane Product distribution / selectivity; | |
| In water Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| In water at 20℃; for 1h; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| With hydrogen; 5%-palladium/activated carbon In ethanol at 30℃; under 3677.86 Torr; for 3h; Product distribution / selectivity; |

-
-
75-64-9
tert-butylamine

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| In water Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| In 2,2-dimethoxy-propane at 25 - 30℃; for 4 - 5h; Product distribution / selectivity; | |
| at 25 - 30℃; for 4 - 5h; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| Stage #1: (2S,3aS,7aS)-1-{2-[1-(ethoxycarbonyl)-(S)-butylamine]-(S)-propionyl}octahydroindole-2-carboxylic acid 4-methoxybenzyl ester With hydrogen; palladium on activated charcoal In ethyl acetate under 2068.65 Torr; for 5h; Stage #2: tert-butylamine In ethyl acetate at 45℃; for 2h; |
-
-
200423-22-9
(2S,3aS,7aS)-octahydroindole-2-carboxylic acid trimethylsilyl ester

-
-
869877-95-2
N-[1(S)-ethoxycarbonyl-1-butyl]-(S)-alanyl chloride hydrochloride

-
-
75-64-9
tert-butylamine

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Stage #1: N-[1(S)-ethoxycarbonyl-1-butyl]-(S)-alanyl chloride hydrochloride With 1H-imidazole In dichloromethane at 10 - 15℃; for 1h; Stage #2: (2S,3aS,7aS)-octahydroindole-2-carboxylic acid trimethylsilyl ester In dichloromethane at 10℃; for 3h; Stage #3: tert-butylamine In dichloromethane at 10 - 40℃; for 1h; Product distribution / selectivity; |

-
-
869877-95-2
N-[1(S)-ethoxycarbonyl-1-butyl]-(S)-alanyl chloride hydrochloride

-
-
80875-98-5
(2S,3aS,7aS)-perhydroindole-2-carboxylic acid

-
-
75-64-9
tert-butylamine

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Stage #1: N-[1(S)-ethoxycarbonyl-1-butyl]-(S)-alanyl chloride hydrochloride With 1H-imidazole In dichloromethane at -5 - 0℃; for 1h; Stage #2: (2S,3aS,7aS)-perhydroindole-2-carboxylic acid In dichloromethane at -5 - 25℃; for 4.75h; Stage #3: tert-butylamine In dichloromethane at 10 - 40℃; for 1h; Product distribution / selectivity; |

-
-
86647-57-6
(2S,3aS,7aS)-phenylmethyl octahydroindole-2-carboxylate hydrochloride

-
-
82834-12-6
N-<1(S)-(ethoxycarbonyl)butyl>-L-alanine

-
-
75-64-9
tert-butylamine

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Stage #1: (2S,3aS,7aS)-phenylmethyl octahydroindole-2-carboxylate hydrochloride; N-<1(S)-(ethoxycarbonyl)butyl>-L-alanine With triethylamine In Isopropyl acetate at 10 - 15℃; for 0.25h; Stage #2: With 2,4,6-tripropyl-1,3,5,2,4,6-trioxatriphosphinane-2,4,6-trioxide In Isopropyl acetate at -10 - 30℃; for 6h; Stage #3: tert-butylamine With hydrogen; 5% palladium over charcoal In ethanol |

-
-
79815-20-6
(S)-indoline-2-carboxylic acid

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydrogen; 8 % Pd/C / methanol / 55 - 65 °C / 37503.8 Torr / Autoclave 2.1: triethylamine; chloro-trimethyl-silane / dichloromethane / 2 h / 20 °C / Inert atmosphere 2.2: 4 h / -5 - 5 °C 3.1: ethyl acetate / 0.5 h View Scheme |
-
-
89-98-5
2-chloro-benzaldehyde

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: sodium acetate; acetic anhydride / 0.5 h / 20 °C / Inert atmosphere 1.2: 90 - 100 °C 2.1: sodium / 40 - 50 °C / Inert atmosphere 3.1: hydrogen; [Rhcod(S)-DuanPhos]BF4 / ethanol / 45 - 55 °C / 15001.5 Torr / Autoclave 4.1: potassium carbonate; copper(l) chloride / dimethyl sulfoxide / 100 - 105 °C / Inert atmosphere 5.1: sulfuric acid; water / Reflux 6.1: hydrogen; 8 % Pd/C / methanol / 55 - 65 °C / 37503.8 Torr / Autoclave 7.1: triethylamine; chloro-trimethyl-silane / dichloromethane / 2 h / 20 °C / Inert atmosphere 7.2: 4 h / -5 - 5 °C 8.1: ethyl acetate / 0.5 h View Scheme |

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: sodium / 40 - 50 °C / Inert atmosphere 2.1: hydrogen; [Rhcod(S)-DuanPhos]BF4 / ethanol / 45 - 55 °C / 15001.5 Torr / Autoclave 3.1: potassium carbonate; copper(l) chloride / dimethyl sulfoxide / 100 - 105 °C / Inert atmosphere 4.1: sulfuric acid; water / Reflux 5.1: hydrogen; 8 % Pd/C / methanol / 55 - 65 °C / 37503.8 Torr / Autoclave 6.1: triethylamine; chloro-trimethyl-silane / dichloromethane / 2 h / 20 °C / Inert atmosphere 6.2: 4 h / -5 - 5 °C 7.1: ethyl acetate / 0.5 h View Scheme |

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: hydrogen; [Rhcod(S)-DuanPhos]BF4 / ethanol / 45 - 55 °C / 15001.5 Torr / Autoclave 2.1: potassium carbonate; copper(l) chloride / dimethyl sulfoxide / 100 - 105 °C / Inert atmosphere 3.1: sulfuric acid; water / Reflux 4.1: hydrogen; 8 % Pd/C / methanol / 55 - 65 °C / 37503.8 Torr / Autoclave 5.1: triethylamine; chloro-trimethyl-silane / dichloromethane / 2 h / 20 °C / Inert atmosphere 5.2: 4 h / -5 - 5 °C 6.1: ethyl acetate / 0.5 h View Scheme |

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: potassium carbonate; copper(l) chloride / dimethyl sulfoxide / 100 - 105 °C / Inert atmosphere 2.1: sulfuric acid; water / Reflux 3.1: hydrogen; 8 % Pd/C / methanol / 55 - 65 °C / 37503.8 Torr / Autoclave 4.1: triethylamine; chloro-trimethyl-silane / dichloromethane / 2 h / 20 °C / Inert atmosphere 4.2: 4 h / -5 - 5 °C 5.1: ethyl acetate / 0.5 h View Scheme |
-
-
110592-39-7
(S)-N-acetyl-2,3-dihydroindoline-2-carboxylic acid methyl ester

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sulfuric acid; water / Reflux 2.1: hydrogen; 8 % Pd/C / methanol / 55 - 65 °C / 37503.8 Torr / Autoclave 3.1: triethylamine; chloro-trimethyl-silane / dichloromethane / 2 h / 20 °C / Inert atmosphere 3.2: 4 h / -5 - 5 °C 4.1: ethyl acetate / 0.5 h View Scheme |
-
-
80875-98-5
(2S,3aS,7aS)-perhydroindole-2-carboxylic acid

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triethylamine; chloro-trimethyl-silane / dichloromethane / 2 h / 20 °C / Inert atmosphere 1.2: 4 h / -5 - 5 °C 2.1: ethyl acetate / 0.5 h View Scheme |
-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| With water In acetone at -80℃; | 100% |
-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| With water at 30 - 40℃; for 240h; Product distribution / selectivity; | 100% |
| With water In acetonitrile at -50 - -45℃; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In dimethyl sulfoxide at 70℃; for 1h; | 88% |
-
-
107133-36-8
perindopril tert-butylamine

-
-
82834-16-0
Perindopril

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane; water at 10 - 30℃; for 0.5h; Product distribution / selectivity; | 80% |
| With hydrogenchloride In dichloromethane; water pH=5.4; Product distribution / selectivity; | |
| Stage #1: perindopril tert-butylamine With sulfuric acid In ethanol at 20 - 25℃; for 1.5h; Stage #2: With sodium hydroxide In dichloromethane; water pH=4.2; Product distribution / selectivity; | |
| With calcium chloride In ethyl acetate Product distribution / selectivity; Heating / reflux; |
-
-
107133-36-8
perindopril tert-butylamine

-
-
95153-31-4
perindoprilat

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 72h; Ambient temperature; | 75% |
| With water; 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide at 59.84℃; for 120h; Kinetics; |
-
-
107133-36-8
perindopril tert-butylamine

-
A
-
129970-98-5
ethyl (2S)-2-[(3S,5aS,9aS,10aS)-3-methyl-1,4-dioxo-5a,6,7,8,9,9a,10,10a-octahydro-3H-pyrazino[1,2-a]indol-2-yl]pentanoate

-
B
-
95153-31-4
perindoprilat

| Conditions | Yield |
|---|---|
| With water at 40℃; Conversion of starting material; | A 0.04% B 0.03% |
-
-
107133-36-8
perindopril tert-butylamine

-
-
67-68-5
dimethyl sulfoxide

-
-
97-00-7
1-chloro-2,4-dinitro-benzene

| Conditions | Yield |
|---|---|
| at 30℃; Activation energy; Further Variations:; Temperatures; |

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| With water at -15℃; for 1h; Product distribution / selectivity; |
-
-
111-87-5
octanol

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| In ethyl acetate at 30 - 60℃; |
-
-
111-70-6
n-heptan1ol

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| In toluene at 20 - 55℃; |
-
-
107133-36-8
perindopril tert-butylamine

-
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| In toluene at 20 - 65℃; | |
| at 20 - 60℃; Product distribution / selectivity; |
-
-
71-41-0
pentan-1-ol

-
-
107133-36-8
perindopril tert-butylamine

| Conditions | Yield |
|---|---|
| In ethyl acetate at 20 - 60℃; |

| Conditions | Yield |
|---|---|
| In ethyl acetate Heating / reflux; |
Perindopril erbumine Chemical Properties
The Molecular Structure of Perindopril erbumine (CAS NO.107133-36-8):
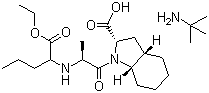
Empirical Formula: C19H32N2O5
Molecular Weight: 368.4678
Nominal Mass: 368 Da
Average Mass: 368.4678 Da
Monoisotopic Mass: 368.231122 Da
Index of Refraction: 1.512
Molar Refractivity: 96.17 cm3
Molar Volume: 320.3 cm3
Surface Tension: 44.9 dyne/cm
Density: 1.15 g/cm3
Flash Point: 278.8 °C
Enthalpy of Vaporization: 89.06 kJ/mol
Boiling Point: 537.4 °C at 760 mmHg
Vapour Pressure: 5.63E-13 mmHg at 25°C
Synonyms: Perindopril ; Perindopril erbumine [USAN] ; (2S,3aS,7aS)-1-((S)-N-((S)-1-Carboxybutyl)alanyl)hexahydro-2-indolinecarboxylic acid, 1-ethyl ester, compound with
tert-butylamine (1:1) ; ACEON ; McN-A-2833-109 ; Perindopril erbumine ; Perindopril tert-butylamine ; S-9490-3 ; UNII-1964X464OJ ; 1H-Indole-2-carboxylic acid, 1-(2-((1-
(ethoxycarbonyl)butyl)amino)-1-oxopropyl)octahydro-, (2S-(1(R*(R*)),2alpha,3abeta,7abeta))-, compd. with 2-methyl-2-propanamine (1:1)
Perindopril erbumine Uses
Perindopril erbumine (CAS NO.107133-36-8) can be used as anangiotensin-converting enzyme (ACE) inhibitor.
Perindopril erbumine Toxicity Data With Reference
| 1. | orl-rat LD :>2500 mg/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 40 (1998),3147. | ||
| 2. | ivn-rat LD50:323 mg/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 40 (1998),3147. | ||
| 3. | orl-mus LD :>2500 mg/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 40 (1998),3147. | ||
| 4. | ivn-mus LD50:323 mg/kg | YAKUD5 Gekkan Yakuji. Pharmaceuticals Monthly. 40 (1998),3147. |
Perindopril erbumine Safety Profile
A poison by intravenous route. Moderately toxic by ingestion. When heated to decomposition it emits toxic vapors of NOx Hazard codes: Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
S36: Wear suitable protective clothing
Related Products
- Perindopril
- Perindopril erbumine
- Perindoprilat
- 107134-59-8
- 1071-37-0
- 1071-39-2
- 107-14-2
- 1071428-42-6
- 1071428-43-7
- 1071433-01-6
- 1071-46-1
- 1071496-88-2
- 107-15-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View