-
Name
Phthalonitrile
- EINECS 202-044-8
- CAS No. 91-15-6
- Article Data122
- CAS DataBase
- Density 1.24 g/cm3
- Solubility 50 mg/mL, clear in benzene, 0.56 g/L (25 °C) in water
- Melting Point 137-139 °C(lit.)
- Formula C8H4N2
- Boiling Point 304.6 °C at 760 mmHg
- Molecular Weight 128.133
- Flash Point 153.3 °C
- Transport Information UN 3439 6.1/PG 2
- Appearance off-white to tan powder
- Safety 36/37/39-45-28A
- Risk Codes 25-23/24/25
-
Molecular Structure
-
Hazard Symbols
 T
T
- Synonyms Phthalonitrile(8CI);1,2-Benzodinitrile;1,2-Bis(cyano)benzene;1,2-Dicyanobenzene;NSC17562;o-Benzenedicarbonitrile;o-Benzenedinitrile;o-Cyanobenzonitrile;o-Dicyanobenzene;
- PSA 47.58000
- LogP 1.42996
Synthetic route
-
-
112069-03-1
N,N'-o-phthaloylbis(P,P,P-triphenylphospha-λ5-azene)

-
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 140℃; intramolecular aza-Wittig reaction; | 100% |

| Conditions | Yield |
|---|---|
| With potassium phenolate; palladium diacetate; potassium carbonate; dimethyl cis-but-2-ene-1,4-dioate; triphenylphosphine In N,N-dimethyl-formamide at 120℃; for 24h; Schlenk technique; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With sodium carbonate; potassium ferrocyanide In N,N-dimethyl-formamide at 120℃; for 5h; | 93% |
-
-
17300-02-6
1,2-benzenedimethanamine

-
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| With aluminum oxide In N,N-dimethyl-formamide at 120℃; for 6h; Inert atmosphere; | 92% |
| With [Ru(p-cymene)(pzH-NP)(Cl)]Cl; potassium tert-butylate In toluene at 70℃; for 24h; Schlenk technique; Inert atmosphere; | 68% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 12h; | 91% |
| With potassium carbonate In N,N-dimethyl-formamide at 120℃; for 15h; Schlenk technique; Green chemistry; | 90% |
| With 3% Pd/CeO2; sodium acetate In N,N-dimethyl-formamide; isopropyl alcohol at 65℃; for 10h; Irradiation; | 42% |
| With tri-tert-butyl phosphine; potassium carbonate In N,N-dimethyl-formamide at 120℃; for 24h; Reagent/catalyst; Inert atmosphere; | 75 %Chromat. |

| Conditions | Yield |
|---|---|
| With dichloro(2,2-bis(diphenylphosphino)diphenyl ether)palladium(II); potassium acetate In 1,4-dioxane; water at 90℃; for 1.33333h; Sealed tube; | 91% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In N,N-dimethyl-formamide at 120℃; for 12h; Inert atmosphere; | 91% |
| With sodium acetate In N,N-dimethyl-formamide; isopropyl alcohol at 55℃; Irradiation; Sealed tube; |
-
-
89942-45-0
2-(hydroxymethyl)benzenecorbonitrile

-
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; copper(l) iodide; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In water for 24h; Reflux; Green chemistry; | 90% |
-
-
13506-83-7
[2-(dihydroxyboranyl)phenyl]boronic acid

-
A
-
6295-97-2
4-bromo-benzenesulfonic acid-(4-chloro-anilide)

-
B
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 15h; Catalytic behavior; Reflux; | A n/a B 90% |

-
-
13506-83-7
[2-(dihydroxyboranyl)phenyl]boronic acid

-
A
-
16937-03-4
4-nitro-N-(4-chlorophenyl)benzenesulfonamide

-
B
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 14h; Catalytic behavior; Reflux; | A n/a B 90% |


| Conditions | Yield |
|---|---|
| With ammonium hydroxide; sodium persulfate; sodium iodide; iron(II) chloride In 1,2-dichloro-ethane at 20 - 50℃; for 16h; | 89% |
| With O-(diphenylphosphinyl)hydroxylamine In toluene at 20 - 85℃; chemoselective reaction; | 78% |
| With formic acid; hydroxylamine hydrochloride; silica gel for 0.0333333h; Irradiation; | 72 % Chromat. |
| With K2Co4[WZn3(H2O)2][ZnW9O34]2*53H2O; ammonia; dihydrogen peroxide In water at 20℃; for 6h; | 100 %Spectr. |

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium carbonate; 1,3-bis[(2,6-diisopropyl)phenyl]imidazolinium chloride In N,N-dimethyl acetamide at 120℃; for 3h; | 89% |

| Conditions | Yield |
|---|---|
| at 390 - 400℃; | A 12% B 88% |

-
-
13506-83-7
[2-(dihydroxyboranyl)phenyl]boronic acid

-
-
119986-58-2
N-(4-chlorophenyl)-N-cyano-4-methylbenzenesulfonamide

-
A
-
2903-34-6
4-methyl-N-(4-chlorophenyl)benzenesulfonamide

-
B
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| With Fe3O4/SiO2/(3-chloropropyl)trimethoxysilane/2,2′-(4,4′-(propane-1,3-diyl)bis(piperazine-4,1-diyl))- diethanamine/Pd In acetonitrile for 16h; Catalytic behavior; Reflux; | A n/a B 88% |

-
-
1044569-46-1
1-hydrazinophthalazine

-
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| With ammonium cerium (IV) nitrate In methanol at 20℃; | 87% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; sodium carbonate; 1,3-bis[(2,6-diisopropyl)phenyl]imidazolinium chloride In N,N-dimethyl acetamide at 120℃; for 10h; | 86% |

| Conditions | Yield |
|---|---|
| With potassium phenolate; palladium diacetate; potassium carbonate; dimethyl cis-but-2-ene-1,4-dioate; triphenylphosphine In N,N-dimethyl-formamide at 120℃; for 24h; Schlenk technique; Inert atmosphere; | 86% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium carbonate In N,N-dimethyl-formamide | 84.8% |

| Conditions | Yield |
|---|---|
| tetrakis(triphenylphosphine) palladium(0) In N,N-dimethyl-formamide Substitution; | 83% |

| Conditions | Yield |
|---|---|
| With dmap; 1,1'-bis-(diphenylphosphino)ferrocene; nickel(II) chloride hexahydrate; zinc In acetonitrile at 80℃; for 6h; Schlenk technique; Inert atmosphere; Sealed tube; | 83% |
| With dmap; 1,1'-bis-(diphenylphosphino)ferrocene; nickel(II) chloride hexahydrate; zinc In acetonitrile at 80℃; for 6h; Inert atmosphere; Sealed tube; | 83% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride In glycerol at 90℃; for 9.5h; Green chemistry; | 83% |
| Multi-step reaction with 2 steps 1: hydroxylamine hydrochloride; sodium carbonate / methanol; water / Reflux 2: fluorosulfonyl fluoride; triethylamine / acetonitrile / 0.25 h / 20 °C / Schlenk technique View Scheme |

| Conditions | Yield |
|---|---|
| With copper(l) iodide; potassium iodide at 80℃; for 1h; Inert atmosphere; Sonication; | 80% |
| With sodium carbonate In N,N-dimethyl-formamide at 120℃; for 12h; | 65% |
| With 1-Butylimidazole; copper(l) iodide In toluene at 160℃; for 16h; Inert atmosphere; | 78 %Chromat. |

| Conditions | Yield |
|---|---|
| With 1-Butylimidazole; copper(l) iodide In toluene at 160℃; for 16h; | 78% |
-
-
7787-87-3
(1-chloroethyl)trimethylsilane

-
-
30757-50-7
4-hydroxy-1,2-benzenedicarbonitrile

-
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium carbonate In N,N-dimethyl-formamide | 77% |
-
-
51762-67-5
3-nitrobenzene-1,2-dicarbonitrile

-
-
2344-80-1
Chloromethyltrimethylsilane

-
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium carbonate; sodium nitrite In 1-methyl-pyrrolidin-2-one; N,N-dimethyl acetamide | 74.7% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium carbonate In N,N-dimethyl-formamide | 73% |
-
-
2344-83-4
(3-chloropropyl)trimethylsilane

-
-
116039-49-7
3-hydroxylphthalonitrile

-
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium carbonate In N,N-dimethyl acetamide; dimethyl sulfoxide | 71.2% |
-
-
10442-39-4
tetra-n-butylammonium cyanide

-
-
114650-54-3
C13H9N3S

-
A
-
91804-55-6
2-(phenylthio)benzonitrile

-
B
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 25℃; for 0.75h; Irradiation; | A 13% B 63% |


| Conditions | Yield |
|---|---|
| With tetra-n-butylammonium cyanide In dimethyl sulfoxide at 25℃; for 1h; Irradiation; | A 13% B 63% |
-
-
91-15-6
phthalonitrile

-
-
160956-25-2, 160956-26-3, 160956-27-4, 160956-28-5, 160956-29-6
isoindole-1,3-diylidenediamine

| Conditions | Yield |
|---|---|
| With ammonia; sulfur at 100℃; for 6h; titanium autoclave; var. cat.: sodium hydrosulfide, benzenethiol; add of MeOH; | 100% |
| With ammonia; sodium methylate In methanol 1 h then reflux, 3 h; | 90% |
| With ammonia for 5h; Heating; | 52% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]-undecen-7-ene In ethylene glycol at 35 - 40℃; for 3h; Product distribution; Further Variations:; Temperatures; Solvents; Reagents; UV-irradiation; | 100% |
| With sodium methylate; blue zeolite In methanol at 40℃; for 72h; | 76% |
| With ammonium sulfate; 1,1,1,3,3,3-hexamethyl-disilazane In N,N-dimethyl-formamide at 150℃; for 24h; | 72% |
-
-
2026-48-4
(S)-valinol

-
-
91-15-6
phthalonitrile

-
-
131380-80-8
1,2-bis[(4S)-(4-isopropyl-Δ2-1,3-oxazolin-2-yl)]benzene

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 150℃; for 1h; Microwave irradiation; | 100% |
| With zinc(II) chloride In toluene for 96h; Heating; | 92% |
| With zinc(II) chloride In chlorobenzene for 24h; Heating; | 89% |
| With polyvinylpyrrolidone capped poly [Zn(2,6-bis(2H-1,2,3,4-tetrazol-2-id-5-yl)pyridine)H2O] nanocrystal In chlorobenzene for 15h; Reflux; | 84% |
-
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 55℃; for 1h; | 100% |
-
-
91-15-6
phthalonitrile

-
-
136918-14-4
phthalimide

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; silica gel In chlorobenzene for 5h; Heating; | 100% |
| With 1,3-dimethyl-1H-imidazol-3-ium hydrogen carbonate; water In ethanol at 80℃; for 4h; Catalytic behavior; Green chemistry; chemoselective reaction; | 90% |
| With copper(II) acetate monohydrate In water; acetic acid Reflux; | 71% |
| With sodium tetrahydroborate In ethanol; water at 80℃; for 4h; regioselective reaction; | 94 %Chromat. |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol pyrophoric Ni under water layer added to a methanol soln. containing an equivalent quantity of phthalonitrile and 5 drops of 30% soln of CH3ONa in methanol; flask maintained at 25°C for 24-72 h; blue product separated from unreacted metal by shaking and decanting with the solvent, washed with ethanol in Soxhlet equipment and dried in air; purity of 95-97%; elem. anal.; | 100% |
| With sodium methylate In methanol; water pyrophoric metal:phthalonitrile=1:4 and few drops of CH3ONa under water layer treated for 24 h at 25°C; septd., washed (ethanol), dried in air, elem. anal.; | 100% |
| With sodium methylate In ethanol pyrophoric Ni under water layer added to an ethanol soln. containing an equivalent quantity of phthalonitrile and 5 drops of 30% soln of CH3ONa in methanol; flask maintained at 25°C for 24-72 h; blue product separated from unreacted metal by shaking and decanting with the solvent, washed with ethanol in Soxhlet equipment and dried in air; purity of 95-97%; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol Sonication; Zn added to a methanol soln. containing an equivalent quantity of phthalonitrile and 5 drops of 30% soln of CH3ONa in methanol; flask put into an ultrasonic cleaner and maintained at 50°C for 24-72 h; blue product separated from unreacted metal by shaking and decanting with the solvent, washed with ethanol in Soxhlet equipment and dried in air; purity of 95-97%; elem. anal.; | 100% |
| With sodium methylate In further solvent(s) Sonication; Zn added to an 1-octanol soln. containing an equivalent quantity of phthalonitrile and 5 drops of 30% soln of CH3ONa in methanol; flask put intoan ultrasonic cleaner and maintained at 50°C for 24-72 h; blue product separated from unreacted metal by shaking and decanting with the solvent, washed with ethanol in Soxhlet equipment and dried in air; purity of 95-97%; elem. anal.; | 100% |
| With sodium methylate In methanol Sonication; Zn added to a methanol soln. containing an equivalent quantity of phthalonitrile and 5 drops of 30% soln of CH3ONa in methanol; flask put into an ultrasonic cleaner and maintained at 40°C for 24-72 h; blue product separated from unreacted metal by shaking and decanting with the solvent, washed with ethanol in Soxhlet equipment and dried in air; purity of 95-97%; elem. anal.; | 85% |

-
-
91-15-6
phthalonitrile

-
-
14096-14-1, 187386-26-1
chloro(phthalodinitile)phthalocyaninato(2-)iridate(III)

| Conditions | Yield |
|---|---|
| With NH4I In neat (no solvent) byproducts: [IrI(PDN)Pc(2-)]; boiling (10 min); extraction (acetone), pptn. (HCl, boiling), centrifugation, washing (H2O), drying (vac.); | 100% |
-
-
7446-70-0, 7784-13-6
aluminum (III) chloride

-
-
57-13-6
urea

-
-
91-15-6
phthalonitrile

-
-
14154-42-8, 62905-77-5
chloroaluminum phthalocyanine

| Conditions | Yield |
|---|---|
| With ammonium molybdate at 200℃; for 12h; | 99.26% |

-
-
74-89-5
methylamine

-
-
91-15-6
phthalonitrile

-
-
88988-76-5, 89130-82-5, 93698-08-9, 93698-09-0
1,3-bis(methylimino)-isoindoline

| Conditions | Yield |
|---|---|
| sulfur at 40℃; for 3h; titanium autoclave; | 99% |
| sulfur at 40℃; for 3h; titanium autoclave; var. primary amines; | 99% |
-
-
91-15-6
phthalonitrile

-
-
71515-74-7
5-(2-cyanophenyl)tetrazole

| Conditions | Yield |
|---|---|
| With sodium azide In water at 100℃; for 0.666667h; Reagent/catalyst; | 99% |
| With sodium azide at 120℃; for 0.583333h; Reagent/catalyst; | 99% |
| With sodium azide at 120℃; for 0.0833333h; | 97% |
-
-
12775-96-1, 15158-11-9, 15721-63-8, 16941-75-6, 17493-86-6, 19498-52-3, 20499-83-6, 20499-84-7, 20499-85-8, 20499-86-9, 20573-10-8, 20573-11-9, 21595-51-7, 21595-52-8, 22206-52-6, 26445-28-3, 28959-95-7, 37362-93-9, 39417-05-5, 54603-16-6, 54603-23-5, 54603-32-6, 54603-40-6, 54603-48-4, 54603-81-5, 54603-89-3, 56316-56-4, 95985-91-4, 122297-32-9, 7440-50-8
copper

-
-
91-15-6
phthalonitrile

-
-
147-14-8
copper phthalocyanine

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol Sonication; Cu added to a methanol soln. containing an equivalent quantity of phthalonitrile and 5 drops of 30% soln of CH3ONa in methanol; flask put into an ultrasonic cleaner and maintained at 50°C for 24-72 h; blue product separated from unreacted metal by shaking and decanting with the solvent, washed with ethanol in Soxhlet equipment and dried in air; purity of 95-97%; elem. anal.; | 99% |
| With sodium methylate In ethanol Sonication; Cu added to an ethanol soln. containing an equivalent quantity of phthalonitrile and 5 drops of 30% soln of CH3ONa in methanol; flask put into an ultrasonic cleaner and maintained at 50°C for 24-72 h; blue product separated from unreacted metal by shaking and decanting with the solvent, washed with ethanol in Soxhlet equipment and dried in air; purity of 95-97%; elem. anal.; | 99% |
| With sodium methylate In methanol Sonication; Cu added to a methanol soln. containing an equivalent quantity of phthalonitrile and 5 drops of 30% soln of CH3ONa in methanol; flask put into an ultrasonic cleaner and maintained at 25°C for 24-72 h; blue product separated from unreacted metal by shaking and decanting with the solvent, washed with ethanol in Soxhlet equipment and dried in air; purity of 95-97%; elem. anal.; | 60% |

| Conditions | Yield |
|---|---|
| Stage #1: phthalonitrile With sodium methylate In 5,5-dimethyl-1,3-cyclohexadiene at 5℃; for 6.5h; Stage #2: 4-(2-cyanoacetyl)benzoic acid With acetic acid In 5,5-dimethyl-1,3-cyclohexadiene at 5 - 45℃; for 26h; Solvent; | 99% |
| Stage #1: phthalonitrile With sodium methylate In methanol at 5℃; for 13.5h; Stage #2: 4-(2-cyanoacetyl)benzoic acid With acetic acid In methanol at 20 - 40℃; for 100h; |

-
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| In dichloromethane at 23℃; for 1h; Inert atmosphere; | 99% |

-
-
7446-70-0, 7784-13-6
aluminum (III) chloride

-
-
91-15-6
phthalonitrile

-
-
14154-42-8
chloroaluminum phthalocyanine

| Conditions | Yield |
|---|---|
| With ammonium chloride at 200℃; for 10.5h; Reagent/catalyst; Concentration; | 98.4% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In pentan-1-ol at 136℃; for 5h; Inert atmosphere; | |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In pentan-1-ol at 136℃; for 5h; |
-
-
380896-89-9
1-(pyridine-4-oxy) 3,4-dicyanobenzene

-
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In pentan-1-ol for 18h; Inert atmosphere; Reflux; | 98.2% |

-
-
100-46-9
benzylamine

-
-
91-15-6
phthalonitrile

-
-
32313-76-1
1,3-Bis-[(Z)-benzylimino]-2,3-dihydro-1H-isoindole

| Conditions | Yield |
|---|---|
| sulfur at 40℃; for 1h; | 98% |
-
-
20989-17-7
(2S)-2-phenylglycinol

-
-
91-15-6
phthalonitrile

-
-
131380-82-0
1,2-bis<(4S)-4-phenyl-2-oxazolin-2-yl>benzene

| Conditions | Yield |
|---|---|
| at 150℃; for 1h; Microwave irradiation; | 98% |
| With zinc(II) chloride In chlorobenzene for 24h; Heating; | 66% |

| Conditions | Yield |
|---|---|
| With CuCl2*2H2O for 0.166667h; microwave irradiation; | 98% |
| With CuCl2*2H2O at 190 - 210℃; for 0.25h; microwave irradiation; | 88% |
| With copper diacetate; 1,8-diazabicyclo[5.4.0]undec-7-ene at 140℃; for 0.0833333h; | 76% |
| With 1,1,1,3,3,3-hexamethyl-disilazane; copper(ll) bromide In N,N-dimethyl-formamide at 100℃; for 10h; | 74% |

-
-
91-15-6
phthalonitrile

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol for 5h; Heating; | 98% |
-
-
109-76-2
Trimethylenediamine

-
-
91-15-6
phthalonitrile

-
-
1476807-78-9
2-(1,4,5,6-tetrahydropyrimidin-2-yl)benzonitrile

| Conditions | Yield |
|---|---|
| With bis(2-hydroxy-κO-2-phenylacetato)copper(II); iodine; sodium acetate In toluene at 90℃; for 0.333333h; Time; Microwave irradiation; chemoselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 40℃; for 0.5h; Reagent/catalyst; Inert atmosphere; Microwave irradiation; | 97% |
-
-
107-10-8
propylamine

-
-
91-15-6
phthalonitrile

-
-
93423-55-3
1,3-Bis-[(Z)-propylimino]-2,3-dihydro-1H-isoindole

| Conditions | Yield |
|---|---|
| sulfur for 2h; Heating; | 96% |

| Conditions | Yield |
|---|---|
| In methanol for 5h; Heating; | 96% |

| Conditions | Yield |
|---|---|
| In further solvent(s) SnCl2 and phthalonitrile were added to 1-chloronaphthalene (solvent); brought to reflux over a period of 1 h; was allowed to reflux for 3 h; cooled slowly to room temperature; filtered; washed in benzene in a Soxhlet extractor for 24 h; elem. anal.; | 96% |
-
-
1603-40-3
3-methylpyridin-2-ylamine

-
-
71-48-7, 917-69-1, 5931-89-5, 55881-15-7, 68931-68-0, 68931-69-1, 93029-27-7
cobalt(II) acetate

-
-
91-15-6
phthalonitrile

-
-
79062-08-1
Co(3-MeBPI)2

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; toluene 1,2-dicyanobenzene and 2-amino-3-methylpyridine heated in oil bath to 210°C for 3 h, mixt. cooled to ambient temp., toluene added, temp.increased to 90°C, NaOH in methanol added, Co(OAc)2 in methanol added and mixt. heated for 1 h; mixt. cooled to 40°C, product filtered, washed with methanol, diethyl ether and dried; | 96% |
| With sodium hydroxide In methanol; toluene 1,2-dicyanobenzene and 2-amino-3-methylpyridine heated in oil bath to 210°C for 4 h, mixt. cooled to 210°C., toluene added, NaOH in methanol added and stirred for 5 min, Co(OAc)2 in methanol added and mixt. stirredd for 45 min; mixt. cooled to 40°C, product filtered, washed with methanol, diethyl ether and dried in vacuo at 80°C; |

-
-
1393596-93-4
(2-picolyl)diethylborane dimer

-
-
91-15-6
phthalonitrile

-
-
1393596-99-0
(Et2B)2(1,2-(NHC(CHC5H4N))2C6H4)

| Conditions | Yield |
|---|---|
| In toluene (inert atm.); reaction of boron compd. and 1 equiv. of dicyanobenzene intoluene at 70°C for 4 h; | 96% |
| In toluene at 70℃; for 4h; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 2h; Reflux; | 96% |
-
-
91-15-6
phthalonitrile

-
-
14320-04-8
zinc phthalocyanine

| Conditions | Yield |
|---|---|
| With zinc acetate dihydrate; N,N-dimethyl-formamide at 200℃; for 0.166667h; Microwave irradiation; Sealed tube; | 95% |
| With N-ethyl-N-hydroxy-ethanamine; zinc(II) chloride at 80℃; for 0.25h; | 45% |
Phthalonitrile Chemical Properties
Molecule structure of 1,2-Benzenedicarbonitrile (CAS NO.91-15-6):
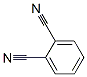
IUPAC Name: Benzene-1,2-dicarbonitrile
Molecular Weight: 128.13076 g/mol
Molecular Formula: C8H4N2
Density: 1.24 g/cm3
Melting Point: 137-139 °C(lit.)
Boiling Point: 304.6 °C at 760 mmHg
Flash Point: 153.3 °C
Index of Refraction: 1.565
Molar Refractivity: 35.88 cm3
Molar Volume: 110 cm3
Polarizability: 14.22×10-24 cm3
Surface Tension: 55.6 dyne/cm
Enthalpy of Vaporization: 54.5 kJ/mol
Vapour Pressure: 0.000865 mmHg at 25 °C
Solubility Benzene: 50 mg/mL, clear
Water Solubility: 0.56 g/L (25 °C)
XLogP3: 1
H-Bond Acceptor: 2
Exact Mass: 128.037448
MonoIsotopic Mass: 128.037448
Topological Polar Surface Area: 47.6
Heavy Atom Count: 10
Complexity: 178
Canonical SMILES: C1=CC=C(C(=C1)C#N)C#N
InChI: InChI=1S/C8H4N2/c9-5-7-3-1-2-4-8(7)6-10/h1-4H
InChIKey: XQZYPMVTSDWCCE-UHFFFAOYSA-N
EINECS: 202-044-8
Product Categories: Industrial/Fine Chemicals; Organics; Functional Materials; Phthalonitriles & Naphthalonitriles; Phthalonitriles (Building Blocks for Phthalocyanines); C8 to C9; Cyanides/Nitriles; Nitrogen Compounds
Phthalonitrile History
In 1896, the first formation of 1,2-Benzenedicarbonitrile was reported by Johannes Pinnow. It was noted to be a byproduct of the reaction between orthamidobenzonitrile hydrochloride, sodium nitrite, and hydrochloric acid to synthesize orthodicyanodiazoamidobenzene. In 1907, the first intentional method derived for the synthesis of 1,2-Benzenedicarbonitrile appeared, when 1,2-Benzenedicarbonitrile was boiled with acetic anhydride. While initial yields were small, it was a precursor that eventually to the contemporary large-scale synthesis seen today.
Phthalonitrile Uses
1,2-Benzenedicarbonitrile (CAS NO.91-15-6) is widely used for the sythesis of phthalein sulfonamide durgs, phthalocyanine pigments and dyes, high-resistance polyamide fiber, xylyl, di-isocyanate plastic and desulfuration catalyst. It is also used for dyes, pigments and other organic synthesis.
Phthalonitrile Production
1,2-Benzenedicarbonitrile is made by the ammoxidation of o-xylene in the presence of a vanadium oxide-antimony-oxide catalyst over heat (480 °C). This process passes a gaseous mixture of ammonia, oxygen, ando-xylene through a distributor plate into a fluidized bed reactor, containing the catalyst.
.gif)
Phthalonitrile Toxicity Data With Reference
| 1. | orl-rat TDLo:7425 mg/kg/66W-I:ETA | VOONAW Voprosy Onkologii. Problems of Onkology. 18 (1)(1972),81. | ||
| 2. | ipr-rat LD50:62 mg/kg | KJMDA6 Kobe Journal of the Medical Sciences. 11 (1965),63. | ||
| 3. | orl-mus LD50:65 mg/kg | INHEAO Industrial Health. 4 (1966),11. | ||
| 4. | ipr-mus LD50:25 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD277-689 . | ||
| 5. | scu-mus LD50:46 mg/kg | INHEAO Industrial Health. 4 (1966),11. |
Phthalonitrile Consensus Reports
Cyanide and its compounds are on the Community Right-To-Know List. Reported in EPA TSCA Inventory.
Phthalonitrile Safety Profile
Hazard Codes:  T
T
Risk Statements: 25-23/24/25
R25 :Toxic if swallowed.
R23/24/25:Toxic by inhalation, in contact with skin and if swallowed.
Safety Statements: 36/37/39-45-28A
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S28:After contact with skin, wash immediately with plenty of soap-suds.
RIDADR: UN 3439 6.1/PG 2
WGK Germany: 1
RTECS: TI8575000
HazardClass: 6.1
PackingGroup: II
HS Code: 29269095
Hazardous Substances Data: 91-15-6(Hazardous Substances Data)
Poison by ingestion, subcutaneous, and intraperitoneal routes. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits toxic fumes of CN− and NOx.
Phthalonitrile Specification
1,2-Benzenedicarbonitrile (CAS NO.91-15-6) is also named as 1,2-Benzodinitrile ; 1,2-Dicyanobenzene ; AI3-00049 ; CCRIS 8898 ; Ftalodinitril ; Ftalodinitril [Czech] ; Ftalonitril ; Ftalonitril [Czech] ; HSDB 5273 ; NSC 17562 ; Phthalic acid dinitrile ; Phthalodinitrile ; Phthalonitrile ; USAF ND-09 ; o-Benzenedicarbonitrile ; o-Benzenedinitrile ; o-Cyanobenzonitrile ; o-Dicyanobenzene ; o-Pdn ; o-Phthalodinitrile ; ortho-Dicyanobenzene . 1,2-Benzenedicarbonitrile (CAS NO.91-15-6) is off-white to tan powder. It is soluble in alcohol, ether, chloroform and benzene, insoluble in oil. It can be volatile with steam. 1,2-Benzenedicarbonitrile is stable and incompatible with strong bases, strong acids, strong oxidizing agents, strong reducing agents.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View