-
Name
Pregnenolone
- EINECS 205-647-4
- CAS No. 145-13-1
- Article Data123
- CAS DataBase
- Density 1.09 g/cm3
- Solubility Insoluble in water
- Melting Point 188-190 °C
- Formula C21H32O2
- Boiling Point 443.3 °C at 760 mmHg
- Molecular Weight 316.484
- Flash Point 188.9 °C
- Transport Information
- Appearance white to off-white powder
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 3-beta-Hydroxypregn-5-en-20-one;3beta-Hydroxy-Delta5-pregnen-20-one;3beta-Hydroxy-5-pregnene-20-one;Delta5-Pregnen-3beta-ol-20-one;Pregn-5-ene-3beta-ol-20-one;Pregnenolone:Pregna-5-ene-3beta-ol-20-one;Arthenolone;
- PSA 37.30000
- LogP 4.51530
Synthetic route

| Conditions | Yield |
|---|---|
| With methanol; sodium In tetrahydrofuran at 20℃; for 4h; Inert atmosphere; | 100% |
| With potassium hydroxide; water In methanol at 30℃; for 2h; | 97% |
| With potassium hydroxide In water; tert-butyl alcohol at 30℃; for 12h; | 96% |

| Conditions | Yield |
|---|---|
| With (1,1',1''-[nitrilotri(4,1-phenylene)]tri(ethan-1-one)); lithium perchlorate; silica gel; sodium hydrogencarbonate In dichloromethane; water; acetonitrile for 2h; electrolysis; | 100% |

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In 1,4-dioxane at 20℃; Hydrolysis; | 97% |
-
-
58701-45-4
3β-t-butyldimethylsilyloxy-pregn-5-en-20-one

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With chloro-methylsulfanyl-methane; water; potassium iodide In 1,4-dioxane at 50℃; for 3.3h; | 97% |
| Multi-step reaction with 5 steps 1.1: n-butyllithium / hexane; tetrahydrofuran / 2.17 h / -78 - -20 °C / Inert atmosphere 1.2: Cooling; Inert atmosphere 2.1: mercury(II) oxide; water; mercury dichloride / acetonitrile / 2.5 h / Reflux 3.1: tetrahydrofuran; diethyl ether / 1.5 h / Cooling 4.1: hydrogenchloride / dichloromethane; methanol; water / 0.25 h 5.1: human P450 11A1; yeast alcohol dehydrogenase; oxygen-18 / aq. phosphate buffer / 37 °C / Microbiological reaction View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: 16-dehydropregnenolone acetate With hydrogen at 0 - 25℃; for 1h; Stage #2: With potassium hydroxide for 2h; Reflux; | 92.8% |
| Multi-step reaction with 2 steps 1: 98 percent / H2 / Pd/C / ethyl acetate / 12 h / 30 °C / 2327.23 Torr 2: 97 percent / potassium hydroxide; H2O / methanol / 2 h / 30 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 97 percent / H2 / Pd/C / 15 h / 30 °C / 2327.17 Torr 2: 96 percent / KOH / 2-methyl-propan-2-ol; H2O / 16 h / 30 °C View Scheme |
-
-
18843-28-2
3β-formyloxypregn-5-en-20-one

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With lipase from Candida cylindracea; octanol In various solvent(s) at 37℃; for 144h; | 90% |
| With bis(tri-n-butyltin)oxide In benzene at 80℃; for 1.5h; | 90% |

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With bis(tri-n-butyltin)oxide In toluene at 110℃; for 0.5h; | 90% |

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With bis(tri-n-butyltin)oxide In toluene at 110℃; for 17h; | 85% |

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide In methanol at 5℃; for 24h; other reagent: lithium, NH3(liquid); | 78% |
-
-
136634-01-0
(20R)-3β,20,26-trihydroxy-27-norcholest-5-en-22-one

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| Stage #1: (20R)-3β,20,26-trihydroxy-27-norcholest-5-en-22-one With hydrazine hydrate In 2,2'-[1,2-ethanediylbis(oxy)]bisethanol at 165 - 195℃; for 2.5h; Stage #2: With sodium periodate; sulfuric acid In ethanol for 0.0833333h; | 37% |
-
-
13057-17-5
bromethyl methyl ether

-
-
73465-46-0
3β,20-diacetoxy-5,17(20)pregnadien

-
A
-
145-13-1
Pregnenolone

-
B
-
82989-16-0
3β-hydroxy-17-methoxymethyl-5-pregnen-20-one

| Conditions | Yield |
|---|---|
| With methyllithium In tetrahydrofuran at 0℃; for 0.0833333h; | A 1.8 g B 36% |

-
-
1778-02-5
Pregnenolone acetate

-
-
129932-29-2
difluoromethyl-diphenyl-phosphine oxide

-
A
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| Stage #1: difluoromethyl-diphenyl-phosphine oxide With lithium diisopropyl amide In tetrahydrofuran at -78℃; for 0.833333h; Stage #2: Pregnenolone acetate In tetrahydrofuran for 1.5h; Wittig reaction; Heating; | A 30.2% B 20% C 13.7% |


| Conditions | Yield |
|---|---|
| In ethanol for 0.5h; mitochondria from the human placenta; | A n/a B 10% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; nickel Hydrogenation; | |
| With Pd-BaSO4; diethyl ether Hydrogenation; |
-
-
917-64-6
methyl magnesium iodide

-
-
57764-90-6
3β-hydroxy-androstene-(5)-carboxylic acid-(17β)-nitrile

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With diethyl ether; benzene Behandeln des eigeengten Reaktionsgemisches mit wss.Saeure,zuletzt unter Erhitzen; |
-
-
996-82-7, 34727-00-9, 73177-21-6
sodium diethylmalonate

-
-
7429-97-2
3β-acetoxy-5-etienic acid chloride

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With benzene anschliessend Behandeln mit Wasser und Erwaermen des Reaktionsprodukts mit methanol.KOH und anschliessend mit wss.-methanol.H2SO4; |
-
-
1236-09-5
pregn-5-ene-3,20-dione

-
A
-
145-13-1
Pregnenolone

-
B
-
19037-28-6
(3α)-3-hydroxypregn-5-en-20-one

| Conditions | Yield |
|---|---|
| With ethanol; nickel Hydrogenation; |
-
-
465-53-2, 15387-47-0, 68138-60-3, 115225-84-8
6β-hydroxy-3α,5α-cyclo-pregnan-20-one

-
A
-
145-13-1
Pregnenolone

-
B
-
474-43-1
3β-fluoro-pregn-5-en-20-one

| Conditions | Yield |
|---|---|
| With hydrogen fluoride |
-
-
911442-53-0
3β-hydroxypregn-5-ene-16,20-dione

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol; zinc |
-
-
60534-25-0
(20S)-3β-acetoxy-5-pregnen-20-amine

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With diethyl ether; hypochloric acid; sodium sulfate Erwaermen des gebildeten Chloramins mit Natriumaethylat in Aethanol und Behandeln des Reaktiongemisches mit Wasser; | |
| With diethyl ether; hypochloric acid; sodium sulfate Erwaermen des gebildeten Chloramins mit Natriumaethylat in Aethanol und Behandeln des Reaktiongemisches mit Wasser; | |
| With diethyl ether; hypochloric acid; sodium sulfate Erwaermen des gebildeten Chloramins mit Natriumaethylat in Aethanol und Behandeln des Reaktiongemisches mit Wasser; |
-
-
112790-35-9
3β-acetoxy-5-chloro-5α-pregnan-20-one

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With methanol; potassium carbonate |
-
-
33769-71-0
21-diazo-3β-hydroxy-pregn-5-en-20-one

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With hydrogenchloride; ethanol; sodium iodide |
-
-
108516-75-2
3β-acetoxy-24,24-diphenyl-chola-5,20(22)ξ,23-triene

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide; chloroform; acetic acid at 0℃; Erwaermen des Reaktionsprodukts mit K2CO3 und wss.Methanol; |
-
-
1474-14-2
3β-acetoxybisnor-5-cholenic acid

-
-
546-67-8
lead(IV) tetraacetate

-
-
64-19-7
acetic acid

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| Erwaermen des Reaktionsprod. mit meth. Kalilauge unter Stickstoff und Behandeln der danach isolierten Saeure mit Blei(IV)-acetat in Essigsaeure; |

-
-
85541-82-8, 95119-09-8, 7254-03-7
methyl 5-androsten-3β-ol-17β-carboxylate

-
-
141-78-6
ethyl acetate

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With sodium Behandeln des Reaktionsgemisches mit Eis und wss.HCl und Erhitzen des Reaktionsprodukts mit wss.-aethanol.NaOH; |
-
-
60-29-7
diethyl ether

-
-
53-43-0
dehydroepiandrosterone

-
-
141-52-6
sodium ethanolate

-
-
535-13-7
2-chloro-propanoic acid, ethyl ester

-
A
-
145-13-1
Pregnenolone


-
C
-
566-63-2
3β-hydroxy-17α-pregn-5-en-20-one

| Conditions | Yield |
|---|---|
| Erhitzen des Reaktionsprodukts mit wss.-aethanol.NaOH; |

-
-
53-43-0
dehydroepiandrosterone

-
-
535-13-7
2-chloro-propanoic acid, ethyl ester

-
A
-
145-13-1
Pregnenolone

-
B
-
566-63-2
3β-hydroxy-17α-pregn-5-en-20-one

| Conditions | Yield |
|---|---|
| With diethyl ether; sodium ethanolate Erhitzen des Reaktionsprodukts mit wss.-aethanol.NaOH; |


| Conditions | Yield |
|---|---|
| at 200℃; Erhitzen des Stereoisomeren-Gemisches; stereoisomer(ic) of mp: 248 degree; | |
| at 200℃; Erhitzen des Steroisomeren-Gemisches; stereoisomer(ic) of mp: 187 degree; |


| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen; acetic acid In tetrahydrofuran at 60℃; under 3102.97 Torr; | 100% |
| With palladium 10% on activated carbon; hydrogen In ethanol for 5h; Inert atmosphere; | 97% |
| palladium-carbon In ethanol | 96% |
-
-
145-13-1
Pregnenolone

-
-
6192-84-3
1-((3S,8S,9S,10R,13S,14S,17R)-3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethan-1-one oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride In pyridine | 100% |
| With pyridine; hydroxylamine hydrochloride In ethanol at 130℃; for 1h; Inert atmosphere; | 95% |
| With hydroxylamine hydrochloride; sodium acetate In ethanol; water Reflux; | 62% |

| Conditions | Yield |
|---|---|
| With pyridine at 37℃; Inert atmosphere; | 100% |
| With pyridine for 60h; Ambient temperature; | 99% |
| With dmap; triethylamine at 25℃; for 0.916667h; | 99% |
-
-
145-13-1
Pregnenolone

-
-
107-30-2
chloromethyl methyl ether

-
-
23328-05-4
3β-methoxymethylether-pregnane-5-ene-20-one

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 2h; Ambient temperature; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; Cooling with ice; | 98% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; Inert atmosphere; Cooling with ice; | 95% |
-
-
145-13-1
Pregnenolone

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
58701-45-4
3β-t-butyldimethylsilyloxy-pregn-5-en-20-one

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane; N,N-dimethyl-formamide for 25h; Ambient temperature; | 100% |
| With 1H-imidazole In dichloromethane | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide | 100% |
-
-
145-13-1
Pregnenolone

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
1526930-28-8
1-((3S,10R,13S,17S)-3-((tert-butyldiphenylsilyl)oxy)-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethanone

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 0 - 50℃; | 100% |
| With 1H-imidazole In dichloromethane at 0 - 20℃; for 4h; | 85% |

| Conditions | Yield |
|---|---|
| With pyridine at 40℃; for 24h; | 99.9% |
-
-
145-13-1
Pregnenolone

-
-
59042-34-1
3β,20-dihydroxypregn-5-ene

| Conditions | Yield |
|---|---|
| With hydrogen In ethanol at 100℃; under 15001.5 Torr; Autoclave; | 99% |
| With sodium tetrahydroborate In tetrahydrofuran; methanol at 0℃; for 0.666667h; | 28 g |
| With sodium tetrahydroborate; cerium(III) chloride heptahydrate In methanol Luche Cerium Reduction; |
-
-
24680-50-0, 71277-11-7, 1963-36-6
4-methoxy-trans-cinnamaldehyde

-
-
145-13-1
Pregnenolone

-
-
1246397-34-1
(2E)-1-((10R,13S)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3(β)-hydroxy-10,13-dimethyl-1H-cyclopenta[α]phenanthren-17(β)-yl)-3-(4-methoxyphenyl)prop-2-en-1-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 20℃; for 12h; Aldol condensation; | 99% |
-
-
145-13-1
Pregnenolone

-
-
555-16-8
4-nitrobenzaldehdye

-
-
1569482-86-5
(E)-3β-hydroxy-21-(4-nitrobenzal)pregn-5-en-20-one

| Conditions | Yield |
|---|---|
| With Aliquat 336; sodium hydroxide In water at 80℃; for 2h; Reagent/catalyst; Time; Aldol Condensation; | 99% |
-
-
145-13-1
Pregnenolone

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
17879-91-3
(3S,8S,9S,10R,13S,14S,17R)-10,13-dimethyl-17-(prop-1-en-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 15 - 50℃; Inert atmosphere; Stage #2: Pregnenolone In tetrahydrofuran at 50 - 65℃; for 1h; Inert atmosphere; | 99% |
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 20 - 50℃; for 0.5h; Inert atmosphere; Stage #2: Pregnenolone In tetrahydrofuran at 50℃; for 1h; | 99% |
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 0℃; for 1h; Heck Reaction; Sealed tube; Inert atmosphere; Stage #2: Pregnenolone In tetrahydrofuran at 0 - 20℃; for 16h; Sealed tube; Reflux; | 97% |
-
-
145-13-1
Pregnenolone

-
-
27200-84-6
methyl triphenylphosphonium bromide

-
-
17879-91-3
(3S,8S,9S,10R,13S,14S,17R)-10,13-dimethyl-17-(prop-1-en-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-ol

| Conditions | Yield |
|---|---|
| Stage #1: methyl triphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 15 - 50℃; for 0.5h; Inert atmosphere; Stage #2: Pregnenolone In tetrahydrofuran at 50 - 65℃; for 1h; Inert atmosphere; | 99% |
-
-
14371-10-9
(E)-3-phenylpropenal

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water for 1h; Inert atmosphere; | 99% |
-
-
145-13-1
Pregnenolone

-
-
37610-80-3
1,3-dioxolan-2-ylethylmagnesium bromide

-
-
86476-21-3
24,24-ethylenedioxy-Δ5-chol-3β,20-diol

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 1h; Ambient temperature; | 98.2% |
-
-
145-13-1
Pregnenolone

-
-
100-52-7
benzaldehyde

-
-
120793-61-5
(2E)-1-((10R,13S)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3(β)-hydroxy-10,13-dimethyl-1H-cyclopenta[α]phenanthren-17(β)-yl)-3-phenylprop-2-en-1-one

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 20℃; for 0.333333h; | 98% |
| With potassium hydroxide In ethanol at 20℃; Claisen Schmidt condensation; | 92% |
| With aluminum oxide; potassium fluoride In ethanol for 2h; Reflux; | 90% |
-
-
145-13-1
Pregnenolone

-
-
98-59-9
p-toluenesulfonyl chloride

-
-
6885-40-1
3β-toluene-4-sulfonyloxy-pregn-5-en-20-one

| Conditions | Yield |
|---|---|
| With pyridine for 14h; | 98% |
| With pyridine; dmap at 20℃; for 12h; | 98% |
| With pyridine at 30℃; for 4h; Tosylation; | 94% |
-
-
145-13-1
Pregnenolone

-
-
85552-32-5
1-((3S,8S,9S,10R,13S,14S,17S)-3-Hydroxy-10,13-dimethyl-hexadecahydro-20-oxa-cyclopropa[5,6]cyclopenta[a]phenanthren-17-yl)-ethanone

| Conditions | Yield |
|---|---|
| With sodium carbonate; 3-chloro-benzenecarboperoxoic acid In dichloromethane | 98% |
| With methyl hexanoate; CpLIP2 Y179F (ipase/acyltransferase) from Candida parapsilosis; dihydrogen peroxide In aq. phosphate buffer; water at 20℃; pH=6.5; Enzymatic reaction; | 39% |
| With magnesium bis(monoperoxyphthalate)hexahydrate In acetone at 57℃; for 0.5h; |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid for 20h; Heating; | 98% |
-
-
421-83-0
trifluoromethane sulfonyl chloride

-
-
145-13-1
Pregnenolone

-
-
58701-45-4
3β-t-butyldimethylsilyloxy-pregn-5-en-20-one

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: oxalyl dichloride; Pregnenolone In diethyl ether at 20℃; for 18h; Stage #2: With water In diethyl ether at 20℃; for 1h; | 98% |
| With triethylamine; N,N-dimethyl-formamide In dichloromethane at 0 - 10℃; for 2h; | 36% |
-
-
145-13-1
Pregnenolone

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
58701-45-4
1-((3S,8S,9S,10R,13S,14S,17S)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-3-[(1,1-dimethylethyl)dimethylsilyloxy]-10,13-dimethyl-1H-cyclopenta[a]phenanthren-17-yl)ethanone

| Conditions | Yield |
|---|---|
| Stage #1: Pregnenolone With 1H-imidazole In N,N-dimethyl-formamide for 0.333333h; Stage #2: tert-butyldimethylsilyl chloride In N,N-dimethyl-formamide at 20℃; for 12h; | 98% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water for 1h; Inert atmosphere; | 98% |
-
-
39511-08-5
(E)-3-(2-furanyl)-2-propenal

-
-
145-13-1
Pregnenolone

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water for 1h; Inert atmosphere; | 98% |
-
-
145-13-1
Pregnenolone

-
-
124-63-0
methanesulfonyl chloride

-
-
38759-50-1
3β-methanesulfonyloxypregn-5-en-20-one

| Conditions | Yield |
|---|---|
| With pyridine at 20 - 25℃; for 4h; Inert atmosphere; | 97.2% |
| With pyridine at 20℃; for 23h; | |
| With pyridine | |
| With triethylamine In toluene |
-
-
13154-24-0
triisopropylsilyl chloride

-
-
145-13-1
Pregnenolone

-
-
241139-90-2
3β-((triisopropylsilyl)oxy)pregn-5-en-20-one

| Conditions | Yield |
|---|---|
| With 1H-imidazole In dichloromethane; N,N-dimethyl-formamide at 20℃; for 4h; Inert atmosphere; | 97% |
| With 1H-imidazole In dichloromethane; N,N-dimethyl-formamide at 25℃; for 24h; | 74% |
-
-
145-13-1
Pregnenolone

-
-
60562-58-5
(E)-1-((3S,8S,9S,10R,13S,14S,17S)-3-hydroxy-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)ethanone oxime

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate In methanol; water at 20℃; for 6h; Inert atmosphere; Reflux; | 97% |
| With hydroxylamine hydrochloride; sodium acetate In methanol; water for 6h; Reflux; | 97% |
| With hydroxylamine hydrochloride; sodium acetate In methanol; water for 6h; Reflux; | 97% |
Pregnenolone Chemical Properties
Molecule structure of Pregnenolone (CAS NO.145-13-1):
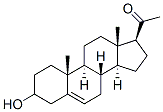
IUPAC Name: 1-[(3S,8S,9S,10R,13S,14S,17S)-3-Hydroxy-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-17-yl]ethanone
Molecular Weight: 316.47758 g/mol
Molecular Formula: C21H32O2
Density: 1.09 g/cm3
Melting Point: 188-190 °C
Boiling Point: 443.3 °C at 760 mmHg
Flash Point: 188.9 °C
Index of Refraction: 1.549
Molar Refractivity: 92.35 cm3
Molar Volume: 290 cm3
Polarizability: 36.61×10-24 cm3
Surface Tension: 42.7 dyne/cm
Enthalpy of Vaporization: 80.89 kJ/mol
Vapour Pressure: 1.02E-09 mmHg at 25 °C
XLogP3: 4.2
H-Bond Donor: 1
H-Bond Acceptor: 2
Rotatable Bond Count: 1
Tautomer Count: 3
Exact Mass: 316.24023
MonoIsotopic Mass: 316.24023
Topological Polar Surface Area: 37.3
Heavy Atom Count: 23
Canonical SMILES: CC(=O)C1CCC2C1(CCC3C2CC=C4C3(CCC(C4)O)C)C
Isomeric SMILES: CC(=O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CC=C4[C@@]3(CC[C@@H](C4)O))C
InChI: InChI=1S/C21H32O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h4,15-19,23H,5-12H2,1-3H3/t15-,16-,17+,18-,19-,20-,21+/m0/s1
InChIKey: ORNBQBCIOKFOEO-QGVNFLHTSA-N
EINECS: 205-647-4
Product Categories: Steroids; Steroids & Hormones - 13C & 2H; Biochemistry; Hydroxyketosteroids; Steroids (Others)
Pregnenolone Uses
Pregnenolone (CAS NO.145-13-1) is used for biochemical research. It is also used as steroid pharmaceutical intermediates and synthesis of steroid drugs.
Pregnenolone Production
Pregnenolone is made by cholesterol. This conversion involves hydroxylation at the side-chain at C20 and C22 positions, with cleavage of the side-chain. The enzyme performing this task is cytochrome P450scc, located in the mitochondria, and controlled by pituitary tropic hormones, such as ACTH, FSH, LH.
Pregnenolone Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD | intraperitoneal | > 200mg/kg (200mg/kg) | Journal of Medicinal Chemistry. Vol. 11, Pg. 117, 1968. |
Pregnenolone Safety Profile
Safety Statements: 22-24/25
S22:Do not breathe dust.
S24/25:Avoid contact with skin and eyes.
WGK Germany: 3
RTECS: TU5560700
Pregnenolone Specification
Pregnenolone (CAS NO.145-13-1) is also named as 3-beta-Hydroxypregn-5-en-20-one ; 3beta-Hydroxypregn-5-en-20-one ; 5-Pregnen-3-beta-ol-20-one ; 5-Pregnen-3beta-ol-20-one ; Arthenolone ; Bina-Skin ; Enelone ; NSC 1616 ; Natolone ; Pregn-5-en-20-one, 3-hydroxy-, (3-beta)- ; Pregnenolona ; Pregnenolona [INN-Spanish] ; Pregnenolonum ; Pregnenolonum [INN-Latin] ; Pregnetan ; Pregneton ; Pregnolon ; Prenolon ; Regnosone ; Skinostelon ; UNII-73R90F7MQ8 ; delta5-Pregnenolone . Pregnenolone (CAS NO.145-13-1) is white to off-white powder. It is insoluble in water. Its esterified version, pregnenolone sulfate, is water-soluble.
Related Products
- Pregnenolone
- Pregnenolone acetate
- 14513-34-9
- 145137-38-8
- 145147-04-2
- 145148-39-6
- 145149-48-0
- 145149-50-4
- 145-15-3
- 145155-23-3
- 145157-87-5
- 145158-71-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View