-
Name
Pyridine hydrochloride
- EINECS 211-027-4
- CAS No. 628-13-7
- Article Data170
- CAS DataBase
- Density 1.34 g/cm3
- Solubility 85 g/100 mL in water
- Melting Point 145-147 °C(lit.)
- Formula C5H6ClN
- Boiling Point 115.3 °C at 760 mmHg
- Molecular Weight 115.562
- Flash Point 20 °C
- Transport Information
- Appearance white to tan crystals
- Safety 26-36-37/39
- Risk Codes 20/21/22-36/37/38-22
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Pyridine,hydrochloride (6CI,8CI,9CI);Pyridine, compd. with hydrogen chloride (1:1);Pyridinium chloride;Pyridinium monohydrochloride;
- PSA 12.89000
- LogP 1.88360
Synthetic route
-
-
110-86-1
pyridine

-
-
31562-43-3
tert-butylsulfinyl chloride

-
A
-
628-13-7
pyridine hydrochloride

-
B
-
67-64-1
acetone

-
D
-
75-65-0
tert-butyl alcohol

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide In chloroform at 20℃; for 2h; Further byproducts given; | A 100% B 20% C 40% D 55% |

-
-
31562-43-3
tert-butylsulfinyl chloride

-
-
68409-52-9
tert-butyl hydrodisulfide

-
A
-
62383-66-8
tert-butylsulfenic tert-butylsulfinic dithioanhydride

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With pyridine In chloroform at 20℃; for 2h; | A 90% B 100% |

-
-
54562-14-0
z,z,z-octadeca-6,9,12-trienoyl chloride

-
-
56-81-5
glycerol

-
A
-
628-13-7
pyridine hydrochloride

-
B
-
14465-68-0
tri-γ-linolenin

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 20 - 35℃; | A n/a B 96.3% |
| Stage #1: z,z,z-octadeca-6,9,12-trienoyl chloride; glycerol With pyridine In dichloromethane at 35℃; Stage #2: dmap In dichloromethane at 20℃; Product distribution / selectivity; | A n/a B 65% |


| Conditions | Yield |
|---|---|
| With chlorosulfonic acid In dichloromethane at 0℃; for 0.5h; | A n/a B 95% |
-
-
110-86-1
pyridine

-
-
95058-77-8
2-deoxy-2,2-difluoro-1-carbonylribose

-
-
75-36-5
acetyl chloride

-
A
-
946424-26-6
2-deoxy-2,2-difluoro-D-erythro-pentofuranos-1-ulose-3,5-diacetate

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With dmap In ethyl acetate at 60 - 65℃; | A 94% B n/a |

-
-
933-88-0
ortho-toluoyl chloride

-
-
58-61-7
adenosine

-
A
-
628-13-7
pyridine hydrochloride

-
B
-
104579-36-4
N6,2',3',5'-tetra-O-toluoyladenosine

-
C
-
527-85-5
o-Methylbenzamid

| Conditions | Yield |
|---|---|
| With methanol; ammonia In pyridine at 0℃; | A n/a B 93% C n/a |

-
-
110-86-1
pyridine

-
-
866108-64-7
(2Z,4R,5R)-3-amino-4,5-dimethyl-oct-2-enoic acid ethyl ester

-
-
75-36-5
acetyl chloride

-
A
-
866108-66-9
(2Z,4R,5R)-3-acetylamino-4,5-dimethyl-oct-2-enoic acid ethyl ester

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| In dichloromethane at -20 - 0℃; for 2.5h; | A 93% B n/a |

-
-
110-86-1
pyridine

-
-
95058-77-8
2-deoxy-2,2-difluoro-1-carbonylribose

-
-
103-80-0
phenylacetyl chloride

-
A
-
946424-25-5
2-deoxy-2,2-difluoro-D-erythro-pentofuranos-1-ulose-3,5-bis(phenylacetate)

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With dmap In ethyl acetate at 60 - 65℃; | A 92.9% B n/a |


| Conditions | Yield |
|---|---|
| With acetyl chloride In methanol; diethyl ether at 0℃; for 1h; Inert atmosphere; | 92% |
| With chlorosulfonic acid In 1,2-dichloro-ethane at 25℃; Thermodynamic data; ΔH(formation of product); | |
| With hydrogenchloride In 1,2-dichloro-ethane at 25℃; Thermodynamic data; ΔH(formation of product); |
-
-
112-13-0
n-decanoyl chloride

-
-
56-81-5
glycerol

-
A
-
621-71-6
capric acid triglyceride

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: n-decanoyl chloride; glycerol With pyridine In dichloromethane at 20 - 35℃; for 0.0833333 - 0.25h; Stage #2: With dmap at 20℃; | A 86% B n/a |

-
-
78775-72-1
2-tert-butylmalonyl dichloride

-
-
540-51-2
2-bromoethanol

-
A
-
628-13-7
pyridine hydrochloride

-
B
-
78775-97-0
tert-Butylmalonsaeure-bis(2-bromethylester)

| Conditions | Yield |
|---|---|
| With pyridine In benzene for 41h; | A n/a B 83% |

-
-
4342-61-4
1,2-dichlorotetramethylsilane

-
A
-
17865-73-5
octamethyl-1,4-dioxa-2,3,5,6-tetrasilacyclohexane

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With pyridine; nickel dihydroxide In toluene; acetonitrile Heating; | A 69% B 70.6% C n/a |

-
-
62147-49-3
1,3-dihydroxyacetone dimer

-
-
112-13-0
n-decanoyl chloride

-
A
-
73312-67-1
1,3-dicaproyloxypropan-2-one

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 20 - 30℃; | A 60% B n/a |

-
-
17598-93-5
1, 3-dicaprin

-
-
54562-14-0
z,z,z-octadeca-6,9,12-trienoyl chloride

-
A
-
847019-77-6
glycerol 1,3-didecanoate 2-octadecatri(6-Z, 9-Z, 12-Z)enoate

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 10 - 20℃; | A 56% B n/a |


| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 10 - 20℃; | A 56% B n/a |

-
-
110-86-1
pyridine

-
-
122-01-0
4-chloro-benzoyl chloride

-
-
95058-77-8
2-deoxy-2,2-difluoro-1-carbonylribose

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With dmap In ethyl acetate at 60 - 65℃; for 370h; | A 54.1% B n/a |

-
-
2465-32-9, 98168-52-6
1,3-diolein

-
-
54562-14-0
z,z,z-octadeca-6,9,12-trienoyl chloride

-
A
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 10 - 20℃; | A n/a B 54% |

-
-
57303-04-5
arachidonoyl chloride

-
-
56-81-5
glycerol

-
A
-
628-13-7
pyridine hydrochloride

-
B
-
23314-57-0
1,2,3-tris[(cis,cis,cis,cis)-5,8,11,14-eicosatetraenoyl]glycerol

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 25 - 42℃; for 2h; Neat (no solvent); | A n/a B 43% |

-
-
62147-49-3
1,3-dihydroxyacetone dimer

-
-
112-77-6
(Z)-9-octadecenoyl chloride

-
A
-
24472-44-4
2-oxopropane-1,3-diyl dioleate

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 20℃; | A 27% B n/a |

-
-
118773-91-4
tetraethylammonium pentachloro(pyridine)iridate(IV)

-
-
75-18-3
dimethylsulfide

-
-
50-81-7
ascorbic acid

-
A
-
118773-83-4
tetraethylammonium pentachloro(dimethylsulphide)iridate(IV)

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With HCl; chlorine In ethanol; dichloromethane Ir-compd. dissolved in warm ethanol, dropwise addn. of ascorbic acid in ethanol, addn. of ligand in CH2Cl2, refluxed for 1 h, ethanol evapd. in vac., dissolved in CH2Cl2, oxidised with chlorine, saturated with HCl, after 1 h purged with nitrogen; partioned with water, organic phase separated and dried over Na2SO4, reduced in vac., addn. of diethyl ether, left at -10°C, liquid decanted off, dried in vac.; elem. anal.; | A 20% B n/a |

-
-
118773-91-4
tetraethylammonium pentachloro(pyridine)iridate(IV)

-
-
593-79-3
dimethylselenide

-
-
50-81-7
ascorbic acid

-
A
-
118773-79-8
tetraethylammonium pentachloro(dimethylselenide)iridate(IV)

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With HCl; chlorine In ethanol; dichloromethane Ir-compd. dissolved in warm ethanol, dropwise addn. of ascorbic acid in ethanol, addn. of ligand in CH2Cl2, refluxed for 1 h, ethanol evapd. in vac., dissolved in CH2Cl2, oxidised with chlorine, saturated with HCl, after 1 h purged with nitrogen; partioned with water, organic phase separated and dried over Na2SO4, reduced in vac., addn. of diethyl ether, left at -10°C, liquid decanted off, dried in vac.; elem. anal.; | A 20% B n/a |

-
-
118773-91-4
tetraethylammonium pentachloro(pyridine)iridate(IV)

-
-
50-81-7
ascorbic acid

-
A
-
118773-77-6
tetraethylammonium pentachloro(triphenylphosphine)iridate(IV)

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With P(C6H5)3; chlorine; HCl In ethanol; dichloromethane Ir-compd. dissolved in warm ethanol, dropwise addn. of ascorbic acid in ethanol, addn. of ligand in CH2Cl2, refluxed for 1 h, ethanol evapd. in vac., dissolved in CH2Cl2, oxidised with chlorine, saturated with HCl, after 1 h purged with nitrogen; partioned with water, organic phase separated and dried over Na2SO4, reduced in vac., addn. of diethyl ether, left at -10°C, liquid decanted off, dried in vac.; elem. anal.; | A 20% B n/a |

-
-
118773-91-4
tetraethylammonium pentachloro(pyridine)iridate(IV)

-
-
603-32-7
triphenyl-arsane

-
-
50-81-7
ascorbic acid

-
A
-
118773-89-0
tetraethylammonium pentachloro(triphenylarsine)iridate(IV)

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With HCl; chlorine In ethanol; dichloromethane Ir-compd. dissolved in warm ethanol, dropwise addn. of ascorbic acid in ethanol, addn. of ligand in CH2Cl2, refluxed for 1 h, ethanol evapd. in vac., dissolved in CH2Cl2, oxidised with chlorine, saturated with HCl, after 1 h purged with nitrogen; partioned with water, organic phase separated and dried over Na2SO4, reduced in vac., addn. of diethyl ether, left at -10°C, liquid decanted off, dried in vac.; elem. anal.; | A 20% B n/a |

-
-
118773-91-4
tetraethylammonium pentachloro(pyridine)iridate(IV)

-
-
603-36-1
triphenylantimony

-
-
50-81-7
ascorbic acid

-
A
-
118773-85-6
tetraethylammonium pentachloro(triphenylstibine)iridate(IV)

-
B
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With HCl; chlorine In ethanol; dichloromethane Ir-compd. dissolved in warm ethanol, dropwise addn. of ascorbic acid in ethanol, addn. of ligand in CH2Cl2, refluxed for 1 h, ethanol evapd. in vac., dissolved in CH2Cl2, oxidised with chlorine, saturated with HCl, after 1 h purged with nitrogen; partioned with water, organic phase separated and dried over Na2SO4, reduced in vac., addn. of diethyl ether, left at -10°C, liquid decanted off, dried in vac.; elem. anal.; | A 20% B n/a |


-
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.25h; | 100% |
-
-
930606-42-1
5-methoxy-N-[1-(pyridin-2-ylmethyl)-1H-indazol-5-yl]quinazolin-4-amine

-
-
628-13-7
pyridine hydrochloride

-
-
930606-35-2
4-{[1-(pyridin-2-ylmethyl)-1H-indazol-5-yl]amino}quinazolin-5-ol

| Conditions | Yield |
|---|---|
| Stage #1: 5-methoxy-N-[1-(pyridin-2-ylmethyl)-1H-indazol-5-yl]quinazolin-4-amine; pyridine hydrochloride In pyridine for 3h; Heating / reflux; Stage #2: With sodium hydrogencarbonate In water for 0.5h; Product distribution / selectivity; | 100% |
-
-
99589-88-5
trimethylsilylimidovanadium(V)-trichloride

-
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| In toluene all manipulations under Ar; pyridinium salt added to soln. of V compd. with stirring, suspn. stirred for 12 h; filtered, washed with toluene, dried in vac.; elem. anal.; | 98% |

-
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With air In 1,4-dioxane at 20℃; | 98% |
| In 1,4-dioxane at 20℃; for 24h; Inert atmosphere; | 70 mg |
-
-
628-13-7
pyridine hydrochloride

-
-
18820-83-2
pyridine hydroiodide

| Conditions | Yield |
|---|---|
| With methyl iodide In acetonitrile Heating; | 97% |

-
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| at 20℃; | 97% |

-
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With air In 1,4-dioxane at 20℃; | 97% |
-
-
110-86-1
pyridine

-
-
13450-90-3
Gallium trichloride

-
-
628-13-7
pyridine hydrochloride

-
-
72794-64-0
pyridine-pyridinium tetrachlorogallate(III)

| Conditions | Yield |
|---|---|
| In pyridine (N2); soln. of GaCl3 in pyridine and soln. of C5H5NHCl in pyridine were combined; after 1 h pyridine removed (vac.); residue crystd. (toluene); elem. anal.; | 95% |


-
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| at 20℃; | 95% |

-
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| With air In 1,4-dioxane at 20℃; | 95% |
-
-
134695-26-4
tris(2,6-dimethylphenylimino)methylrhenium(VII)

-
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| In dichloromethane addn. of educts; stirred for 1 h at room temp.;; solvent concd. in vacuo; mixed with hexane; elem. anal.;; | 94% |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; pyridine byproducts: H2NC6H3(CH(CH3)2)2, NaCl; addn. of 22.8 mmol of the Na(THF)2 salt to a stirred slurry of 105 mmol (C5H5NH)Cl in 50 ml THF / 200 ml C5H5N, concn. to 30 ml after 10 min., addn. of 300 ml pentane, washing, drying and refluxing with 150 ml C6H6, 5 days, concn. and addn. of pentane;; drying in vacuum; detn. by elem. anal., (1)H-NMR- and (13)C-NMR spectroscopy;; | 94% |


-
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| In water | 93% |

-
-
628-13-7
pyridine hydrochloride

| Conditions | Yield |
|---|---|
| In methanol; dichloromethane CH2Cl2 son. of complex was added to MeOH soln. of Py*HCl; standing 1 d at room temp.; treatment with hexane. cooling; | 92% |
Pyridine hydrochloride Consensus Reports
Pyridine hydrochloride Specification
This chemical has the IUPAC name Pyridine hydrochloride, and it is also known as Pyridinium chloride. Its molecular formula is C5H6ClN and its molecular weight is 115.56. The CAS registry number of this chemical is 628-13-7. Additionally, its product categories are Heterocyclic Compounds; Pyridinium Compounds.
Other characteristics of the Pyridine hydrochloride can be summarised as followings: (1)ACD/LogP: 0.73; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.54; (4)ACD/LogD (pH 7.4): 0.72; (5)ACD/BCF (pH 5.5): 1.37; (6)ACD/BCF (pH 7.4): 2.08; (7)ACD/KOC (pH 5.5): 38.58; (8)ACD/KOC (pH 7.4): 58.76; (9)#H bond acceptors: 1; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 3.88 Å2; (13)Flash Point: 20 °C; (14)Enthalpy of Vaporization: 35.09 kJ/mol; (15)Boiling Point: 115.3 °C at 760 mmHg; (16)Vapour Pressure: 22.8 mmHg at 25°C.
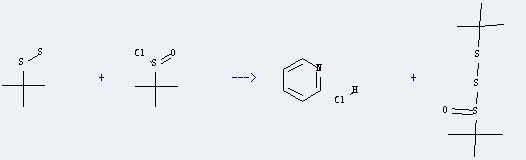
Production method of the Pyridine hydrochloride: It could be obtained by the reactants of 2-methyl-2-propane-sulfinyl chloride and tert-butyl-disulfane. This reaction needs the reagent of pyridine, and the solvent of CHCl3. The yield is 90 %. In addition, this reaction should be taken for 2 hours at the temperature of 20 °C.
Uses of the Pyridine hydrochloride: It's used as pharmaceutical intermediates, organic synthesis and it's suitable for pure gum rubber. It's also suitable for rubber containing carbon black. For example, it could react with thiocyanic acid; potassium salt to obtain the pyridine; thiocyanate. This reaction needs the solvent of H2O. The yield is 95 %. In addition, this reaction needs the heating.
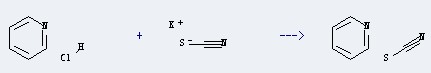
When you are using this chemical, please be cautious about it as the following: This chemical is irritating / harmful to eyes, respiratory system and skin. You should wear suitable protective clothing if you use it. In case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1.SMILES: [Cl-].[nH+]1ccccc1
2.InChI: InChI=1/C5H5N.ClH/c1-2-4-6-5-3-1;/h1-5H;1H
3.InChIKey: AOJFQRQNPXYVLM-UHFFFAOYAN
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| guinea pig | LD50 | skin | 2500uL/kg (2.5mL/kg) | Kodak Company Reports. Vol. 21MAY1971, | |
| rat | LD50 | intraperitoneal | 800mg/kg (800mg/kg) | Kodak Company Reports. Vol. 21MAY1971, | |
| rat | LD50 | oral | 1600mg/kg (1600mg/kg) | Kodak Company Reports. Vol. 21MAY1971, |
Related Products
- Pyridine
- Pyridine hydrobromide
- Pyridine hydrochloride
- Pyridine hydrofluoride
- Pyridine iodine monochloride complex
- Pyridine methanesulfonate
- Pyridine sulfur trioxide
- Pyridine trifluoroacetate
- Pyridine, 2-(2,4-difluorophenoxy)-3-nitro-
- Pyridine, 2-(3-azetidinyl)- (9CI)
- 6281-42-1
- 628-16-0
- 628-17-1
- 6282-02-6
- 628-20-6
- 628-21-7
- 62821-89-0
- 6282-24-2
- 628-22-8
- 62823-14-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View