-
Name
5-Methyluridine
- EINECS 215-973-9
- CAS No. 1463-10-1
- Article Data71
- CAS DataBase
- Density 1.576 g/cm3
- Solubility
- Melting Point 183-184 °C(lit.)
- Formula C10H14N2O6
- Boiling Point
- Molecular Weight 258.231
- Flash Point
- Transport Information
- Appearance white powder
- Safety 24/25
- Risk Codes 26/27/28
-
Molecular Structure
-
Hazard Symbols
 T+
T+
- Synonyms 1-[(2R,3R,4R,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methyl-pyrimidine-2,4-dione;Uridine,5-methyl-;5-Methyl uridine;Thymine riboside;Methyluridine;5-Methyluridin, Ribothymidine;
- PSA 124.78000
- LogP -2.54350
Synthetic route

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol | 98% |
| With ammonia In methanol for 96h; | 98% |
| With sodium methylate In methanol for 0.5h; Heating; | 93% |
-
-
3180-76-5
2',3',5'-tri-O-benzoyluridine

-
A
-
93-89-0
benzoic acid ethyl ester

-
B
-
55-21-0
benzamide

-
C
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With ammonia In ethanol at 20℃; | A n/a B n/a C 95% |

-
-
101796-28-5
1-(5'-O-trityl-β-D-ribofuranosyl)thymine

-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With carbon tetrabromide In methanol at 20℃; for 12h; Irradiation; | 94% |
| With sulfuric acid; water; silica gel In acetonitrile at 25℃; for 0.283333h; | 93% |
-
-
119794-52-4
1-[5'-O-(tert-butyldimethylsilyl)-β-D-ribofuranosyl]thymine

-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With chloro-methylsulfanyl-methane; water; potassium iodide In 1,4-dioxane at 50℃; for 5.3h; | 93% |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; C17H20N4O9P(1-)*Na(1+); oxygen In water; acetonitrile at 20℃; under 760.051 Torr; for 7h; Irradiation; chemoselective reaction; | 88% |
-
-
87817-95-6
2',3'-O-isopropylidene-5'-O-methoxymethyl-5-methyluridine

-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid for 19h; Ambient temperature; | 87% |
-
-
144490-03-9
1,2,3,5-tetra-O-acetyl-β-L-ribofuranose

-
-
7288-28-0
2,4-bis(trimethylsiloxy)-5-methylpyrimidine

-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| Stage #1: 1,2,3,5-tetra-O-acetyl-β-L-ribofuranose; 2,4-bis(trimethylsiloxy)-5-methylpyrimidine With tin(IV) chloride In dichloromethane for 12h; Reflux; Stage #2: With acetic acid In dichloromethane at 20℃; for 1h; Stage #3: With ammonia In methanol at 20℃; for 12h; | 85.06% |

| Conditions | Yield |
|---|---|
| With purine nucleoside phosphorylase; uridine phosphorylase at 90℃; for 48h; pH=9; aq. phosphate buffer; Enzymatic reaction; | 85% |
| In water; dimethyl sulfoxide at 25℃; pH=7.4; aq. phosphate buffer; Enzymatic reaction; | 79.1% |
-
-
4336-39-4
(2R,3R,4R,5R)-2-(acetoxymethyl)-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-3,4-diyl diacetate

-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 20℃; for 6h; Inert atmosphere; Schlenk technique; | 81% |
| With ammonia In methanol at 20℃; | |
| With Amberlite IRA 120 H+ In 1,4-dioxane; methanol | 14.1 g |
-
-
1391764-47-8
5-(2,5-dimethoxythiophenyl)-5-methyl-5,6-dihydrouridine

-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| In water for 0.0666667h; Photolysis; | 78% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; xanthine oxidase; purine and pyrimidine nucleoside phosphorylase (PUNP, PYNP) at 40℃; for 11h; | 76% |
| With potassium phosphate; xanthine oxidase; PUNP, PYNP at 40℃; for 11h; Equilibrium constant; | 76% |

| Conditions | Yield |
|---|---|
| With potassium dihydrogenphosphate; E. coli purine nucleoside phosphorylase; E. coli uridine phosphorylase In aq. buffer at 20℃; for 20h; pH=7.5; Enzymatic reaction; | 75% |

| Conditions | Yield |
|---|---|
| With osmium(VIII) oxide In pyridine; N,N-dimethyl-formamide; benzene for 1.5h; Ambient temperature; | 74% |

| Conditions | Yield |
|---|---|
| With mutant Escherichia coli uridine phosphorylase Lys235Arg, Gln236Ala; purine nucleoside phosphorylase from Bacillus halodurans at 60℃; for 2h; pH=7; Kinetics; Reagent/catalyst; Temperature; aq. buffer; Enzymatic reaction; | A n/a B 73.1% |


-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With nickel In ethanol Heating; | 65% |
-
-
64898-15-3
2',3',5'-tris-O-(tert-butyldimethylsilyl)uridine

-
-
74-88-4
methyl iodide

-
A
-
16710-13-7
6-methyluridine

-
B
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction; | A n/a B 61% |
| Yield given. Multistep reaction; | A 4% B n/a |


| Conditions | Yield |
|---|---|
| BM-11 E. coli cells, 0.015 M potassium phosphate buffer (pH 7.25); 1.) 60 deg C, 2 h, 2.) 4 deg C, 18 h; | 45% |

| Conditions | Yield |
|---|---|
| uridine phosphorylase; | 45% |
-
-
214039-19-7
(S)-2',3'-O-(2-bromo-1-diethylphosphonoethylidene)-5'-O-tert-butyldiphenylsilyl-5-methyluridine

-
B
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With potassium fluoride In N,N-dimethyl-formamide at 130℃; for 6h; Ring cleavage; desilylation; | A 35% B 39% |
-
-
175875-24-8
5,S2-Dimethyl-2',3',5'-tri-O-acetyl-2-thiouridine

-
A
-
1463-10-1
5-Methyluridine

-
B
-
29789-17-1
2,5'-Anhydro-5-methyluridine

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 55℃; | A 25% B 33% |
-
-
30414-00-7
5-(hydroxymethyl)uridine

-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With acetic acid; platinum Hydrogenation; |
-
-
75-24-1
trimethylaluminum

-
-
111582-94-6
1-((2R,3R,4R,5R)-3,4-Bis-trimethylsilanyloxy-5-trimethylsilanyloxymethyl-tetrahydro-furan-2-yl)-5-bromo-4-trimethylsilanyloxy-1H-pyrimidin-2-one

-
A
-
58-96-8
uridine

-
B
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With water; ammonium chloride; triphenylphosphine 1) THF, reflux, 10 h, 2) MeOH, reflux, 3 h; Yield given. Multistep reaction. Yields of byproduct given; | |
| With tetrakis(triphenylphosphine) palladium(0); water; ammonium chloride 1) THF, reflux, 2.5 h, 2) MeOH, reflux, 3 h; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
75-24-1
trimethylaluminum

-
-
111582-84-4
1-((2R,3R,4R,5R)-3,4-Bis-trimethylsilanyloxy-5-trimethylsilanyloxymethyl-tetrahydro-furan-2-yl)-5-iodo-4-trimethylsilanyloxy-1H-pyrimidin-2-one

-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With water; ammonium chloride; triphenylphosphine 1) THF, reflux, 4 h, 2) MeOH, reflux, 3 h; Yield given. Multistep reaction; |
-
-
73-97-2
orotate anion

-
A
-
73-97-2
2,6-Dioxo-1,2,3,6-tetrahydro-pyrimidine-4-carboxylic acid anion

-
B
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| In water at 20℃; Rate constant; Equilibrium constant; pH 8.5; |


| Conditions | Yield |
|---|---|
| In water at 20℃; Rate constant; Equilibrium constant; pH 8.4-8.7; |


| Conditions | Yield |
|---|---|
| In water at 20℃; Rate constant; Equilibrium constant; pH 8.4-8.7; |


| Conditions | Yield |
|---|---|
| With trifluoroacetic acid Yield given; | |
| Multi-step reaction with 5 steps 1.1: 5% rhodium on alumina; hydrogen / methanol / 12 h / 4654.46 Torr 2.1: pyridine; silver nitrate; triethylamine / tetrahydrofuran / 4 h 3.1: lithium diisopropyl amide / tetrahydrofuran / 0.5 h / -78 °C 3.2: 0.42 h / -78 °C 4.1: trifluoroacetic acid / water / 1.5 h 5.1: water / 0.07 h / Photolysis View Scheme |

| Conditions | Yield |
|---|---|
| In water at 20℃; Rate constant; pH 8.4-8.7; |


| Conditions | Yield |
|---|---|
| In water at 20℃; Rate constant; Equilibrium constant; pH 8-8.5, var. pH; |

-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
1463-10-1
5-Methyluridine

-
-
134821-75-3
5′-O-tert-butyldiphenylsilyl-O2-2′-anhydro-5-methyluridine

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 20℃; for 18h; Inert atmosphere; | 100% |
| With pyridine at 20℃; for 24h; silylation; | |
| With 1H-imidazole In N,N-dimethyl-formamide Molecular sieve; Inert atmosphere; | |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 4h; Inert atmosphere; |
-
-
69304-37-6
1,3-Dichloro-1,1,3,3-tetraisopropyldisiloxane

-
-
1463-10-1
5-Methyluridine

-
-
130983-87-8
1-[(6aR,8R,9S,9aR)-tetrahydro-9-hydroxy-2,2,4,4-tetraisopropyl-6H-furo[3,2-f]-[1,3,5,2,4]trioxadisilocin-8-yl](5-methylpyrimidine)-2.4(1H.3H)-dione

| Conditions | Yield |
|---|---|
| With pyridine for 6h; Ambient temperature; | 98% |
| In pyridine for 15h; Ambient temperature; | 84% |
| With pyridine | 80% |
-
-
506-96-7
Acetyl bromide

-
-
1463-10-1
5-Methyluridine

-
-
110483-43-7
1-(3,5-di-O-acetyl-2-bromo-2-deoxy-β-D-ribofuranosyl)thymine

| Conditions | Yield |
|---|---|
| In acetonitrile Heating; | 97% |
| With Trimethyl orthoacetate; hydrogen bromide 1.) acetic acid, 50 deg C, 1 h, 2.) 50 - 60 deg C, 5 h; Yield given. Multistep reaction; |
-
-
108-24-7
acetic anhydride

-
-
1463-10-1
5-Methyluridine

-
-
4336-39-4
(2R,3R,4R,5R)-2-(acetoxymethyl)-5-(5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)tetrahydrofuran-3,4-diyl diacetate

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In acetonitrile for 1h; Ambient temperature; | 97% |
| With pyridine for 10h; Ambient temperature; | 94% |
| With pyridine; dmap at 20℃; for 12h; | 90% |
| With pyridine at 0℃; for 5h; |
-
-
84416-80-8
Diethyl-phosphoramidous acid (2R,3S,5R)-2-[bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-5-(5-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-tetrahydro-furan-3-yl ester methyl ester

-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With 1H-tetrazole; silica gel In acetonitrile for 0.25h; | 97% |
-
-
124-63-0
methanesulfonyl chloride

-
-
1463-10-1
5-Methyluridine

-
-
99614-96-7
2′,3′,5′-tris(methanesulfonyl)-5-methyluridine

| Conditions | Yield |
|---|---|
| With 4-methyl-morpholine In acetone at 5 - 50℃; for 2.5h; | 97% |
| With triethylamine In dichloromethane at 20℃; for 0.00416667h; Reagent/catalyst; Temperature; Sonication; Flow reactor; | 97% |
| With 4-methyl-morpholine In acetone |

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 10h; Reflux; | 97% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethylene glycol monomethyl ether-3-methylimidazolium methanesulfonate at 25 - 35℃; | 96% |
| With dmap In various solvent(s) at 25 - 30℃; for 1.5h; | 96% |
| With pyridine; dmap at 40℃; for 3h; | 89% |
| With triethylamine In 1,4-dioxane at 20℃; for 0.666667h; | 70% |
-
-
1463-10-1
5-Methyluridine

-
-
22423-26-3
O-2,2'-cyclo-5-methyluridine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Diethyl carbonate In N,N-dimethyl-formamide at 120℃; for 8h; Reagent/catalyst; | 95.6% |
| With bis(phenyl) carbonate; sodium hydrogencarbonate In N,N-dimethyl-formamide at 100℃; | 90% |
| With bis(phenyl) carbonate; sodium hydrogencarbonate In DMF (N,N-dimethyl-formamide) for 1h; Heating / reflux; | 85% |
-
-
78635-91-3
Dimethyl-phosphoramidous acid (2R,3S,5R)-2-[bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]-5-(5-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-tetrahydro-furan-3-yl ester methyl ester

-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With 1H-tetrazole; silica gel In acetonitrile for 0.25h; Product distribution; other reagent, other amount of reagent; | 95% |
-
-
77-76-9
2,2-dimethoxy-propane

-
-
1463-10-1
5-Methyluridine

-
-
2073-43-0
2',3'-O,O-isopropylidene-5-methyluridine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 20℃; for 24h; | 95% |
| 92% | |
| With toluene-4-sulfonic acid In acetone at 20℃; | 58% |
-
-
931-94-2
1,1-dimethoxycyclopentane

-
-
1463-10-1
5-Methyluridine

-
-
161824-82-4
1-(2'-O,3'-O-Cyclopentyl-5'-hydroxy-β-D-erythro-pentofuranosyl)-5-methyluracil

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In 1,2-dichloro-ethane at 65℃; for 1.33333h; | 95% |

| Conditions | Yield |
|---|---|
| With acetic acid at 50℃; for 1h; | 95% |

| Conditions | Yield |
|---|---|
| In chloroform; acetic acid | 95% |
-
-
3287-99-8, 39110-74-2
benzylamine hydrochloride

-
-
1463-10-1
5-Methyluridine

-
-
1221295-59-5
(2'R,6'R)-2'-thymin-1-yl-6'-hydroxymethyl-N-benzyl morpholine

| Conditions | Yield |
|---|---|
| Stage #1: benzylamine hydrochloride; 5-Methyluridine With sodium periodate; sodium tetraborate decahydrate In methanol for 2.5h; Stage #2: With sodium cyanoborohydride; triethylamine In methanol for 1h; Stage #3: With trifluoroacetic acid In methanol for 3h; | 95% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; copper(II) sulfate for 48h; sealed bottle; | 94% |
| With sulfuric acid at 0 - 20℃; | 91% |
| With sulfuric acid; copper(II) sulfate | 75% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; orthoformic acid triethyl ester In 1,4-dioxane; N,N-dimethyl-formamide at 20℃; for 25h; | 94% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
1463-10-1
5-Methyluridine

-
-
119794-52-4
1-[5'-O-(tert-butyldimethylsilyl)-β-D-ribofuranosyl]thymine

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 20℃; for 18h; Inert atmosphere; | 93% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; | 85% |
| With 1H-imidazole In N,N-dimethyl-formamide for 20h; | 73% |
| With 1H-imidazole In dichloromethane at 20℃; for 48h; | 50% |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 20℃; for 12h; Inert atmosphere; | 93% |
-
-
107-04-0
1-Bromo-2-chloroethane

-
-
1463-10-1
5-Methyluridine

-
-
1085484-19-0
3-(2-chloroethyl)-2'-hydroxythymidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 0.05h; microwave irradiation; | 92% |
-
-
933-40-4
cycloxexanone dimethyl ketal

-
-
1463-10-1
5-Methyluridine

-
-
1191255-79-4
2',3'-O-cyclohexylidene-5-methyluridine

| Conditions | Yield |
|---|---|
| Stage #1: cycloxexanone dimethyl ketal; 5-Methyluridine With toluene-4-sulfonic acid In tetrahydrofuran for 0.5h; Reflux; Stage #2: With sodium hydrogencarbonate In tetrahydrofuran; water at 20℃; | 92% |
| With camphor-10-sulfonic acid In 1,2-dichloro-ethane for 2h; Reflux; Inert atmosphere; |
-
-
1463-10-1
5-Methyluridine

-
-
149-73-5
trimethyl orthoformate

-
-
79137-02-3
2',3'-di-O-methoxymethylidene-5-methyluridine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In N,N-dimethyl-formamide at 20℃; | 91% |
-
-
85272-31-7
di-tert-butylsilyl bis(trifluoromethanesulfonate)

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
1463-10-1
5-Methyluridine

-
-
922508-15-4
1-[2,2-di-tert-butyl-7-(tert-butyl-dimethyl-silanyloxy)-tetrahydro-furo[3,2-d][1,3,2]dioxasilin-6-yl]-5-methyl-1H-pyrimidine-2,4-dione

| Conditions | Yield |
|---|---|
| Stage #1: di-tert-butylsilyl bis(trifluoromethanesulfonate); 5-Methyluridine In N,N-dimethyl-formamide at 20℃; Stage #2: tert-butyldimethylsilyl chloride With 1H-imidazole In N,N-dimethyl-formamide at 20℃; | 91% |


| Conditions | Yield |
|---|---|
| With dmap In various solvent(s) at 50℃; for 2h; | 90% |
-
-
85272-31-7
di-tert-butylsilyl bis(trifluoromethanesulfonate)

-
-
1463-10-1
5-Methyluridine

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine In N,N-dimethyl-formamide at 0 - 20℃; Inert atmosphere; | 90% |
| With 2,6-dimethylpyridine In N,N-dimethyl-formamide at 0 - 20℃; for 0.5h; Inert atmosphere; | 90% |
Ribosylthymine Chemical Properties
Molecule structure of 5-Methyluridine (CAS NO.1463-10-1):
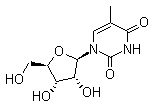
IUPAC Name: 1-[(2R,3R,4S,5R)-3,4-Dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione
Molecular Weight: 258.22796 g/mol
Molecular Formula: C10H14N2O6
Density: 1.576 g/cm3
Melting Point: 183-184 °C(lit.)
Index of Refraction: 1.618
Molar Refractivity: 57.43 cm3
Molar Volume: 163.7 cm3
Surface Tension: 75.9 dyne/cm
Storage Temp.: 2-8 °C
XLogP3: -1.6
H-Bond Donor: 4
H-Bond Acceptor: 6
Rotatable Bond Count: 2
Tautomer Count: 3
Exact Mass: 258.085186
MonoIsotopic Mass: 258.085186
Topological Polar Surface Area: 119
Heavy Atom Count: 18
Canonical SMILES: CC1=CN(C(=O)NC1=O)C2C(C(C(O2)CO)O)O
Isomeric SMILES: CC1=CN(C(=O)NC1=O)[C@H]2[C@@H]([C@@H]([C@H](O2)CO)O)O
InChI: InChI=1S/C10H14N2O6/c1-4-2-12(10(17)11-8(4)16)9-7(15)6(14)5(3-13)18-9/h2,5-7,9,13-15H,3H2,1H3,(H,11,16,17)/t5-,6-,7-,9-/m1/s1
InChIKey: DWRXFEITVBNRMK-JXOAFFINSA-N
EINECS: 215-973-9
Product Categories: Pyridines, Pyrimidines, Purines and Pteredines; Miscellaneous Biochemicals; Nucleotides and Nucleosides; Biochemistry; Nucleosides and their analogs; Nucleosides, Nucleotides & Related Reagents; Bases & Related Reagents; Nucleotides
Ribosylthymine Safety Profile
Hazard Codes:  T+
T+
Risk Statements: 26/27/28
R26/27/28:Very toxic by inhalation, in contact with skin and if swallowed.
Safety Statements: 24/25
S24/25:Avoid contact with skin and eyes.
WGK Germany: 3
Ribosylthymine Specification
5-Methyluridine (CAS NO.1463-10-1) is also named as Ribothymidine ; Thymine riboside ; Uridine, 5-methyl- . 5-Methyluridine (CAS NO.1463-10-1) is white powder. The stability of 5-methyluridine under standard temperature and pressure (STP) is very high.
Related Products
- Ribosylthymine
- 14631-08-4
- 14631-20-0
- 14631-44-8
- 14631-45-9
- 14631-46-0
- 1463-17-8
- 146318-03-8
- 14632-85-0
- 146328-85-0
- 146328-87-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View