-
Name
Sodium phenylpyruvate
- EINECS 204-053-2
- CAS No. 114-76-1
- Article Data5
- CAS DataBase
- Density 1.257g/cm3
- Solubility Soluble in water
- Melting Point >300 °C(lit.)
- Formula C9H8O3.Na
- Boiling Point 299.1 °C at 760 mmHg
- Molecular Weight 186.143
- Flash Point 148.9 °C
- Transport Information
- Appearance White powder
- Safety 22-24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Benzenepropanoicacid, a-oxo-, sodium salt (9CI);Pyruvicacid, phenyl-, sodium salt (8CI);Phenylpyroracemic acid sodium salt;Phenylpyruvic acid sodium salt;Sodium 3-phenyl-2-oxopropionate;b-Phenylpyruvic acid sodium salt;Sodium b-phenylpyruvate;
- PSA 57.20000
- LogP -0.45190
Synthetic route
-
-
93831-17-5
(Z)-5'-benzylidenecyclohexanespiro-2'-(1',3'-dioxolan)-4'-one

-
A
-
108-94-1
cyclohexanone

-
B
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; water for 18h; Ambient temperature; | A n/a B 89% |
-
-
3775-01-7, 74805-60-0, 109754-06-5
5-benzylidenehydantoin

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 140℃; for 3h; Hydrolysis; | 77% |
-
-
63-91-2
L-phenylalanine

-
-
113-24-6
sodium pyruvate

-
A
-
114-76-1
sodium phenylphyruvate

-
B
-
302-72-7
rac-Ala-OH

| Conditions | Yield |
|---|---|
| With acetate buffer; N(1+)C5His2C16; PL(1+)2C16; copper(II) perchlorate In water at 30℃; for 48h; Kinetics; var. catalysts; | A n/a B 27% |

-
-
63-91-2
L-phenylalanine

-
-
22202-68-2
α-ketoglutarate monosodium salt

-
A
-
617-65-2
Glutamic acid

-
B
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| With acetate buffer; N(1+)C5His2C16; PL(1+)2C16; copper(II) perchlorate In water at 30℃; for 48h; Kinetics; |

-
-
38879-46-8
(Z)-4-benzylidene-2-methyl-5(4H)-oxazolone

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium methylate In methanol for 3h; Product distribution; Mechanism; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 76 percent / sodium acetate / acetic acid / 5 h / Heating 2: 77 percent / NaOH / H2O / 3 h / 140 °C View Scheme |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In propan-1-ol; water at 125 - 130℃; under 2250.23 Torr; Temperature; Pressure; Inert atmosphere; |

-
-
75-09-2
dichloromethane

-
-
25599-08-0
tris[(6-methyl-2-pyridyl)methyl]amine

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 5h; | 95% |

-
-
114-76-1
sodium phenylphyruvate

-
-
29700-34-3
<15N>-L-phenylalanine

| Conditions | Yield |
|---|---|
| With formate dehydrogenase; 1,4-dihydronicotinamide adenine dinucleotide; 15N-ammonium formate; phenylalanine dehydrogenase; diothiothreitol Ambient temperature; TRIS, pH=6.5-7.0, 0.1M HCl; | 92% |

-
-
593-56-6
N-methoxylamine hydrochloride

-
-
114-76-1
sodium phenylphyruvate

-
-
154070-59-4
Pd{O2CC(CH2C6H5)N(OCH3)}2

| Conditions | Yield |
|---|---|
| With NaOH In water N2; soln. of sodium-α-oxocarboxylate and O-alkylhydroxylamine stirred at room temp. for 10-24 h; soln. of Na2PdCl4 added slowly;; washed with cold water; dried in high vacuum;; recrystd. from EtOH/H2O; elem. anal.;; | 90% |


| Conditions | Yield |
|---|---|
| at 45 - 65℃; for 15h; Time; Inert atmosphere; | 89.9% |


| Conditions | Yield |
|---|---|
| In methanol at 45 - 65℃; for 24h; Time; Inert atmosphere; | 88.8% |

-
-
67-56-1
methanol

-
-
3290-99-1
4-methoxybenzoic acid hydrazide

-
-
818-08-6
di(n-butyl)tin oxide

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| at 45 - 65℃; for 8h; Inert atmosphere; | 88.5% |


| Conditions | Yield |
|---|---|
| In methanol at 45 - 65℃; for 5h; Inert atmosphere; | 85.5% |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; diethyl ether | 85% |
-
-
67-56-1
methanol

-
-
1135-99-5
diphenyltin(IV) dichloride

-
-
5351-23-5
4-hydroxybenzoic acid hydrazide

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| at 45 - 65℃; for 5h; Inert atmosphere; | 84.5% |

-
-
43100-38-5
4-tert-butylbenzoic acid hydrazide

-
-
70335-32-9
dibenzyltin dichloride

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| In methanol at 50 - 65℃; for 20h; Concentration; Inert atmosphere; | 82.8% |

-
-
114-76-1
sodium phenylphyruvate

-
-
828-01-3, 7326-19-4, 20312-36-1, 156-05-8
D-phenyllactic acid

| Conditions | Yield |
|---|---|
| With phosphate buffer; D-glucose; 2-hydroxyethanethiol; sodium chloride; formate dehydrogenase; 1,4-dihydronicotinamide adenine dinucleotide; benzoylformate reductase; bovine serum albumin; yeast alcohol dehydrogenase In ethanol; water; toluene at 30℃; for 48h; | 80% |
| With hydrogenchloride; Candida boidinii formate dehydrogenase; ethylenediaminetetraacetic acid; Staphylococcus epidermis D-lactate dehydrogenase; NAD; ammonium formate; 2-hydroxyethanethiol In water for 24h; pH=7; Enzymatic reaction; enantioselective reaction; |

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| Stage #1: potassium hydrotris(3-phenyl-5-methyl-pyrazol-1-yl)borate; iron(II) chloride In dichloromethane for 1h; Stage #2: sodium phenylphyruvate In methanol; dichloromethane for 8h; | 80% |


-
-
114-76-1
sodium phenylphyruvate

-
-
618-36-0
rac-methylbenzylamine

-
-
154070-66-3
Pd{O2CC(CH2C6H5)N(CH(CH3)C6H5)}2

| Conditions | Yield |
|---|---|
| In methanol N2;α-oxocarboxylate and amine in MeOH stirred at room temp.; Na2PdCl4 added; stirred at room temp. for 1 h;; evapd.; stirred in hexane; crystd.; washed with hexane and water; dried in high vacuo; elem. anal.;; | 79% |

-
-
64-17-5
ethanol

-
-
3002-01-5
dibenzyltin(IV) dichloride

-
-
936-02-7
2-Hydroxybenzoylhydrazine

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| at 45 - 65℃; for 15h; Concentration; Inert atmosphere; | 78.6% |

-
-
67-56-1
methanol

-
-
3002-01-5
dibenzyltin(IV) dichloride

-
-
613-94-5
benzoic acid hydrazide

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| at 45 - 65℃; for 15h; Concentration; | 78.2% |

-
-
143-66-8
sodium tetraphenyl borate

-
-
16858-01-8
tris[(2-pyridylmethyl)amine]

-
-
114-76-1
sodium phenylphyruvate

-
-
1418284-81-7
[(TPA)CoII(PPH)](BPh4)

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 5h; | 78% |


| Conditions | Yield |
|---|---|
| at 50 - 65℃; for 5h; Time; | 77.9% |

-
-
67-56-1
methanol

-
-
3002-01-5
dibenzyltin(IV) dichloride

-
-
114-76-1
sodium phenylphyruvate

-
-
3619-22-5
p-toluic hydrazide

| Conditions | Yield |
|---|---|
| at 50 - 65℃; for 20h; Concentration; Inert atmosphere; | 77.8% |


-
-
75-09-2
dichloromethane

-
-
143-66-8
sodium tetraphenyl borate

-
-
25599-08-0
tris[(6-methyl-2-pyridyl)methyl]amine

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| In methanol ligands and Fe salt in MeOH stirred under Ar for 2 h; pptd. with a MeOH soln. of NaBPh4, filtered, washed (MeOH), dried; elem.anal.; | 76% |

-
-
1135-99-5
diphenyltin(IV) dichloride

-
-
936-02-7
2-Hydroxybenzoylhydrazine

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| In methanol at 45 - 65℃; for 8h; Concentration; | 75.8% |

-
-
3217-93-4
N-phenylethylhydroxylamine

-
-
114-76-1
sodium phenylphyruvate

-
-
5460-60-6
N-phenethyl-2-phenylacetamide

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide for 15h; Product distribution / selectivity; | 75% |

| Conditions | Yield |
|---|---|
| at 50 - 65℃; for 20h; Inert atmosphere; | 74.5% |

-
-
849144-53-2
bis(2,4-dichlorobenzyl)tin dichloride

-
-
936-02-7
2-Hydroxybenzoylhydrazine

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| In ethanol at 45 - 65℃; for 22h; Concentration; Time; Inert atmosphere; | 74.3% |

-
-
114-76-1
sodium phenylphyruvate

-
-
20312-36-1
L-3-phenyllactic acid

| Conditions | Yield |
|---|---|
| With fructose 1,6-bisphosphate trisodium; nicotinamide adenine dinucleotide red. form; piperazine*HCl buffer; sodium formate; diothiothreitol In water lyophilized formate dehydrogenase, mutant lactate dehydrogenase from Bacillus stearothermophilus (BSLDH), pH=6.0; | 70% |
| With Fructose 1,6-bisphosphate; piperazine*HCl buffer; sodium formate; nicotinamide adenine dinucleotide; diothiothreitol pH 6.0, lyophilized formate dehydrogenase, lactate dehydrogenase from Bacillus stearothermophilus (BSLDH Q102R/C97G); | 65% |
| With fructose 1,6-bisphosphate trisodium; lyophilized formate dehydrogenase; mutant lactate dehydrogenase; nicotinamide adenine dinucleotide red. form; sodium formate; piperazine hydrochloride; diothiothreitol In water at 25℃; Rate constant; other 2-keto acids; other enzymes; stereospecific enzymatic reduction; |

| Conditions | Yield |
|---|---|
| In methanol at 45 - 65℃; for 5h; Time; Inert atmosphere; | 68.5% |

-
-
114-76-1
sodium phenylphyruvate

-
-
74-88-4
methyl iodide

-
-
91133-59-4
3-methyl-2-oxo-3-phenylbutanoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol | 68% |
| With sodium hydroxide In tetrahydrofuran | 65% |
| With sodium hydroxide | 42% |

-
-
34985-37-0
(S)-2-methylbutylamine

-
-
114-76-1
sodium phenylphyruvate

| Conditions | Yield |
|---|---|
| In methanol N2;α-oxocarboxylate and amine in MeOH stirred at room temp.; Na2PdCl4 added; stirred at room temp. for 1 h;; evapd.; stirred in hexane; crystd.; washed with hexane and water; dried in high vacuo; elem. anal.;; | 68% |

-
-
114-76-1
sodium phenylphyruvate

-
-
261764-97-0
6,7-diamino-8-chloroquinoline

-
-
1037303-28-8
3-benzyl-5-chloropyrido[2,3-g]quinoxalin-2(1H)-one

| Conditions | Yield |
|---|---|
| Stage #1: sodium phenylphyruvate; 6,7-diamino-8-chloroquinoline With sulfuric acid In water at 60℃; for 1h; Stage #2: With sodium hydroxide In water | 66% |
| With sulfuric acid In water; N,N-dimethyl-formamide at 60℃; for 1h; |
Sodium phenylpyruvate Specification
The Benzenepropanoic acid, a-oxo-, sodium salt (1:1) with CAS registry number of 114-76-1 is also known as Sodium phenylpyruvate. The IUPAC name is Sodium 2-oxo-3-phenylpropanoic acid. Its EINECS registry number is 204-053-2. In addition, the formula is C9H8O3.Na and the molecular weight is 187.14. This chemical is a white powder that may cause inflammation to the skin or other mucous membranes.
Physical properties about Benzenepropanoic acid, a-oxo-, sodium salt (1:1) are: (1)ACD/LogP: 0.54; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -2.3; (4)ACD/LogD (pH 7.4): -3.18; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 3; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 3; (12)Flash Point: 148.9 °C; (13)Enthalpy of Vaporization: 56.92 kJ/mol; (14)Boiling Point: 299.1 °C at 760 mmHg; (15)Vapour Pressure: 0.000546 mmHg at 25 °C.
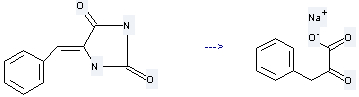
Preparation of Benzenepropanoic acid, a-oxo-, sodium salt (1:1): it is prepared by hydrolysis reaction of 5-benzylidene-imidazolidine-2,4-dione. The reaction needs reagent NaOH and solvent H2O at the temperature of 140 °C for 3 hours. The yield is about 77%.
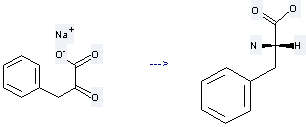
Uses of Benzenepropanoic acid, a-oxo-, sodium salt (1:1): it is used to produce L-phenylalanine. The reaction occurs with reagents (R)-15-amino-methyl-14-hydroxy-5,5-dimethyl-2,8-dithia<9>(2,5)pyridinophane, Zn(ClO4)*6H2O and solvent methanol with other condition of ambient temperature for 24 hours. The yield is about 60%.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing. Do not breathe dust and avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1=CC=C(C=C1)CC(=O)C(=O)O.[Na+]
2. InChI: InChI=1S/C9H8O3.Na/c10-8(9(11)12)6-7-4-2-1-3-5-7;/h1-5H,6H2,(H,11,12);/q;+1
3. InChIKey: MQGYVGKMCRDEAF-UHFFFAOYSA-N
Related Products
- Sodium
- Sodium 2,4-dimethylbenzenesulfonate
- SODIUM γ-FLUORO-β-HYDROXYBUTYRATE
- Sodium ((3-methoxy-1-methyl-3-oxo-1-propenyl)amino)phenylacetate
- Sodium (+)-10-camphorsulfonate
- Sodium (2-carbamoylphenoxy)acetate
- Sodium (2-methyl-4-chlorophenoxy)acetate
- Sodium (C10-16)alkylbenzenesulfonate
- Sodium (R,R)-5-(2-((2-(3-chlorophenyl)-2-hydroxyethyl)amino)propyl)-1,3-benzodioxole-2,2-
- Sodium 1,3-benzothiazole-2-thiolate
- 1147-64-4
- 114772-34-8
- 114772-38-2
- 114772-40-6
- 114772-53-1
- 114772-54-2
- 114772-55-3
- 114776-15-7
- 1147-76-8
- 114780-06-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View