-
Name
Thymidine
- EINECS 200-070-4
- CAS No. 50-89-5
- Article Data200
- CAS DataBase
- Density 1.452 g/cm3
- Solubility soluble in water
- Melting Point 186-188 °C(lit.)
- Formula C10H14N2O5
- Boiling Point 385.05°C (rough estimate)
- Molecular Weight 242.232
- Flash Point
- Transport Information
- Appearance white crystalline powder
- Safety 22-24/25-37/39-26
- Risk Codes 20/21/22-40-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1-(2-Deoxy-beta-D-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione;5-Methyl-2'-deoxyuridine;2,4(1H,3H)-Pyrimidinedione, 1-(2-deoxy-beta-D-erythro-pentofuranosyl)-5-methyl-;2'-Deoxythymidine;5-Methyldeoxyurindine;Uridine, 2'-deoxy-5-methyl-beta-D-Ribofuranoside, thymine-1 2-deoxy-;dT;UNII-VC2W18DGKR;Thyminedeoxyriboside;Thymine-2-deoxyriboside;
- PSA 104.55000
- LogP -1.51430
Synthetic route
-
-
40615-39-2
5'-O-(4-4'-dimethoxytrityl)thymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With zinc dibromide In nitromethane for 0.0166667h; Ambient temperature; | 100% |
| With zinc dibromide In nitromethane Kinetics; Ambient temperature; in various solvent:CH2Cl2, THF, Acetone, Dioxane; other 5'-deoxytritylnucleosides; | 100% |
| With β-naphthol In dichloromethane for 0.166667h; Product distribution / selectivity; Irradiation with mercury arc lamp; | 97% |

| Conditions | Yield |
|---|---|
| With silica gel; trifluoroacetic acid In methanol; chloroform | 100% |
| Stage #1: 5'-O-tritylthymidine With carbon tetrabromide In methanol for 0.5h; Irradiation; Stage #2: In methanol at 20℃; for 12.5h; | 97% |
| With carbon tetrabromide In methanol at 20℃; for 13h; Irradiation; | 97% |
-
-
1335224-06-0
1-[(2R,4S,5R)-5-(hydroxymethyl)-4-[2-(4-hydroxyphenyl)-2-oxoethoxy]tetrahydrofuran-2-yl]-5-methylpyrimidine-2,4-dione

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| In methanol; water for 0.00416667h; Photolysis; | 100% |
-
-
6979-97-1, 97834-40-7, 142405-86-5
3',5'-diacetylthymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With lipase A from Aspergillus niger In aq. phosphate buffer; acetonitrile at 25℃; for 2h; pH=7; Enzymatic reaction; | 99% |
| With ammonium hydroxide at 20℃; Inert atmosphere; | |
| With methanol; sodium methylate at 44℃; under 760.051 Torr; for 1h; Large scale reaction; | 295.6 g |
| With methanol; sodium methylate for 1h; Reflux; |
-
-
40733-26-4
3',5'-O-di(tert-butyldimethylsilyl)thymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide for 1h; Ambient temperature; | 98% |
| With tetrabutyl ammonium fluoride In tetrahydrofuran Ambient temperature; | 95% |
| With acetyl chloride In methanol for 2.5h; | 90% |
-
-
167872-02-8
thymidine 5'-(3'',5''-dimethoxybenzoin)carbonate

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| In benzene for 1h; Irradiation; | 98% |
-
-
82161-35-1
5'-O-(9-phenylthioxanthyl)-2'-deoxythimidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| In water; acetonitrile Hydrolysis; Irradiation; | 97% |
-
-
6979-97-1, 97834-40-7, 142405-86-5
3',5'-diacetylthymidine


-
A
-
35898-31-8
5'-O-acetylthymidine

-
B
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With sodium acetate In acetonitrile at 25℃; for 100h; pH=5; pH-value; Time; Reagent/catalyst; regioselective reaction; | A 97% B 3% |

-
-
40733-28-6
5'-tert-butyldimethylsilyloxy-2'-deoxythymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With Decaborane In tetrahydrofuran; methanol at 20℃; for 6h; | 96% |
| With iodine(I) bromide In methanol; dichloromethane for 2h; Ambient temperature; | 95% |
| With potassium tert-butylate In N,N-dimethyl-formamide for 24h; Ambient temperature; | 92% |

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In aq. phosphate buffer; dimethyl sulfoxide at 20℃; for 0.333333h; pH=7.2; Reagent/catalyst; | 96% |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; C17H20N4O9P(1-)*Na(1+); oxygen In water; acetonitrile at 20℃; under 760.051 Torr; for 7h; Irradiation; chemoselective reaction; | 94% |
-
-
166758-13-0
5'-O-(tert-butyldimethylsilyl)-3'-O-(tert-butyldiphenylsilyl)thymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide for 1.5h; Ambient temperature; | 93% |
-
-
213552-25-1
5'-O-trityl-3'-O-tert-butyldimethylsilyl thymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide for 1h; Ambient temperature; | 93% |
-
-
78687-52-2
Carbonic acid (2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-tetrahydro-furan-2-ylmethyl ester 2-trimethylsilanyl-ethyl ester

-
A
-
74-85-1
ethene

-
B
-
420-56-4
trimethylsilyl fluoride

-
C
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| zinc dibromide In tetrahydrofuran at 20℃; for 0.166667h; deprotection of TMSEC derivat with various catalyst; | A n/a B n/a C 92% |

-
-
42926-80-7
5'-O-(4-monomethoxytrityl)-2'-deoxythymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With cerium(IV) triflate; water In acetonitrile at 25℃; for 0.5h; | 92% |
| With zinc dibromide In nitromethane Kinetics; Ambient temperature; | |
| Multi-step reaction with 7 steps 1: 82 percent / 1.)collidine 2.)I2, H2O / tetrahydrofuran 2: 91 percent 3: 25 percent / 1.)Dowex(Py+) 2.)Ph3P-CCl4 4: 76 percent / NaH/CS2 / dimethylformamide 5: 80 percent / 80percent acetic acid 6: ClCN/H2O/collidine / tetrahydrofuran 7: snake venom phosphodiesterase View Scheme |
-
-
252979-74-1
phenacyl 5'-thymidine carbonate

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With 9,10-dimethylanthracene In acetonitrile for 4h; UV-irradiation; | 91% |
| With N-methylcarbazole In acetonitrile for 4h; UV-irradiation; | 81% |
| In acetonitrile for 3h; UV-irradiation; |
-
-
6979-97-1, 97834-40-7, 142405-86-5
3',5'-diacetylthymidine

-
A
-
21090-30-2
3'-O-acetylthymidine

-
B
-
35898-31-8
5'-O-acetylthymidine

-
C
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With sodium acetate In acetonitrile at 25℃; for 72h; pH=5; regioselective reaction; | A 91% B 3% C 6% |


-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; hydrogen In ethanol; toluene at 45℃; under 2068.65 Torr; for 6h; | 90.9% |
-
-
54898-34-9
2'-chlorothymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With hydrogen; nickel In aq. phosphate buffer at 40℃; under 760.051 Torr; for 8h; pH=7.2; Reagent/catalyst; Temperature; pH-value; Green chemistry; | 90.3% |
-
-
95585-76-5
2'-bromothymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With hydrogen; nickel In methanol under 2327.2 Torr; | 90% |
| With hydrogen; nickel In aq. phosphate buffer at 10 - 20℃; under 760.051 Torr; for 10h; pH=7.4; Green chemistry; | 38.6g |
-
-
118068-35-2
3',5'-bis-O-(tert-butyldiphenylsilyl)-thymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In N,N-dimethyl-formamide for 1h; Ambient temperature; | 89% |
-
-
19083-35-3
5-(benzyloxy)methyl-2′-deoxyuridine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In ethanol at 20℃; for 0.5h; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B; phosphate buffer In 1,4-dioxane at 40℃; for 24h; pH=7; | A 85% B n/a |

| Conditions | Yield |
|---|---|
| With bis(phenyl) carbonate; sodium hydrogencarbonate In water; ethyl acetate; N,N-dimethyl-formamide for 1h; Heating / reflux; | 85% |
| With hydrogenchloride; hydrogen; nickel; carbonic acid dimethyl ester; sodium hydroxide In methanol; N,N-dimethyl acetamide at 50 - 110℃; under 4500.45 Torr; for 28h; Solvent; Reagent/catalyst; Temperature; Pressure; Industrial scale; | |
| Multi-step reaction with 3 steps 1: sodium hydroxide; Diethyl carbonate / N,N-dimethyl-formamide / 8 h / 120 °C 2: hydrogen bromide / N,N-dimethyl-formamide / 4 h / 40 °C 3: hydrogen; nickel / aq. phosphate buffer / 10 h / 10 - 20 °C / 760.05 Torr / pH 7.4 / Green chemistry View Scheme | |
| Multi-step reaction with 3 steps 1: sodium hydroxide; Diethyl carbonate / N,N-dimethyl-formamide / 8 h / 120 °C 2: hydrogenchloride / N,N-dimethyl acetamide / 5 h / 60 °C 3: hydrogen; nickel / aq. phosphate buffer / 8 h / 40 °C / 760.05 Torr / pH 7.2 / Green chemistry View Scheme |
-
-
40733-26-4
3',5'-O-di(tert-butyldimethylsilyl)thymidine

-
A
-
40733-27-5
3'-O-(t-butyldimethylsilyl)thymidine

-
B
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With phosphomolybdic acid In methanol at 20℃; for 3h; Solvent; Reagent/catalyst; regioselective reaction; | A 85% B 9% |
-
-
92447-12-6
3',5'-di-O-methoxyacetylthymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol at 20℃; for 1h; | 84% |
-
-
94189-75-0
N3-benzoyl thymidine

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With benzyl alcohol at 90℃; for 30h; | 82% |
| With ammonium hydroxide for 1h; Ambient temperature; |
-
-
6979-97-1, 97834-40-7, 142405-86-5
3',5'-diacetylthymidine


-
A
-
21090-30-2
3'-O-acetylthymidine

-
B
-
35898-31-8
5'-O-acetylthymidine

-
C
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With sodium acetate In acetonitrile at 25℃; for 48h; pH=5; regioselective reaction; | A 2% B 82% C 16% |

-
-
1220688-60-7
C31H33N3O5

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| With methanol for 1h; UV-irradiation; | 81% |
-
-
108-24-7
acetic anhydride

-
-
50-89-5
thymidine

-
-
6979-97-1, 97834-40-7, 142405-86-5
3',5'-diacetylthymidine

| Conditions | Yield |
|---|---|
| With pyridine for 2h; | 100% |
| With pyridine at 20℃; | 99% |
| With dmap | 98% |

| Conditions | Yield |
|---|---|
| With pyridine; N-ethyl-N,N-diisopropylamine at 20℃; for 0.5h; | 100% |
| With N-ethyl-N,N-diisopropylamine In pyridine for 0.5h; Ambient temperature; | |
| With pyridine at 20℃; |

| Conditions | Yield |
|---|---|
| With subtilisin 8350 In N,N-dimethyl-formamide at 45℃; for 36h; | 100% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
50-89-5
thymidine

-
-
40733-28-6
5'-tert-butyldimethylsilyloxy-2'-deoxythymidine

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 5h; | 100% |
| With silver nitrate In tetrahydrofuran | 98% |
| With dmap In pyridine at 20℃; for 10h; | 97% |
-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
50-89-5
thymidine

-
-
40733-26-4
3',5'-O-di(tert-butyldimethylsilyl)thymidine

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 25℃; | 100% |
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 18h; Inert atmosphere; | 100% |
| With pyridine; 1H-imidazole Inert atmosphere; | 100% |
-
-
58479-61-1
tert-butylchlorodiphenylsilane

-
-
50-89-5
thymidine

-
-
101527-40-6
5'-O-tert-butyldiphenylsilylthymidine

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at -50 - 20℃; for 4h; | 100% |
| With pyridine; dmap for 6h; Ambient temperature; | 95% |
| With dmap In pyridine for 48h; Ambient temperature; | 93% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide; acetone at 20℃; for 72h; | 100% |
-
-
14470-28-1
mono-4-methoxytrityl chloride

-
-
50-89-5
thymidine

-
-
42926-80-7
5'-O-(4-monomethoxytrityl)-2'-deoxythymidine

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In N,N-dimethyl-formamide at 20℃; for 3.5h; | 99% |
| With dmap; triethylamine In N,N-dimethyl-formamide at 20℃; for 4h; Inert atmosphere; | 97% |
| With pyridine | 94% |
-
-
50-89-5
thymidine

-
-
74848-84-3
2'-deoxy-5-(trideuteriomethyl)uridine

| Conditions | Yield |
|---|---|
| With d(4)-methanol; palladium on activated charcoal; hydrogen at 160℃; for 24h; | 99% |
| With deuterium; platinum(IV) oxide In water-d2 at 75 - 80℃; for 72h; | 85% |
| With hydrogen; water-d2; platinum(IV) oxide at 50 - 60℃; for 192h; | 75% |
-
-
50-89-5
thymidine

-
-
25905-51-5
5’-bromo-5’-deoxythymidine

| Conditions | Yield |
|---|---|
| With carbon tetrabromide; triphenylphosphine In N,N-dimethyl acetamide at 20℃; for 5.5h; Appel Halogenation; Inert atmosphere; | 99% |
| With carbon tetrabromide; triphenylphosphine In pyridine for 1h; | 66% |
-
-
13154-24-0
triisopropylsilyl chloride

-
-
50-89-5
thymidine

-
-
54925-58-5
(+)-5-methyl-1-((2R,4S,5R)-4-((triisopropylsilyl)oxy)-5-(((triisopropyl-silyl)oxy)methyl)-tetrahydrofuran-2-yl)pyrimidine-2,4(1H,3H)dione

| Conditions | Yield |
|---|---|
| With 1H-imidazole; dmap In N,N-dimethyl-formamide at 50℃; for 16h; | 99% |

| Conditions | Yield |
|---|---|
| With pyridine In water | 98.6% |
-
-
69304-37-6
1,3-Dichloro-1,1,3,3-tetraisopropyldisiloxane

-
-
50-89-5
thymidine

-
-
97626-18-1
1-[2-deoxy-3,5-O-(1,1,3,3-tetraisopropyldisiloxane-1,3-diyl)-β-D-ribofuranosyl]thymine

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 24h; | 98% |
| With pyridine at 20℃; for 4h; Inert atmosphere; | 95% |
| With pyridine for 0.333333h; Ambient temperature; | 85% |
| With 1H-imidazole In dichloromethane; N,N-dimethyl-formamide at 0℃; for 4h; | 49.9 g |
| With pyridine at 20℃; for 40h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With Rh/Al2O3; hydrogen In methanol; water for 168h; | 98% |
| With hydrogen; Rh/Al2O3 In methanol; water under 2585.7 Torr; for 48h; | 96% |
| With Rh/Al2O3; hydrogen In water at 20℃; under 22502.3 Torr; | 85% |

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In dichloromethane; water at 25℃; for 24h; | 98% |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B In tetrahydrofuran at 60℃; for 63h; | 98% |
| With Candida antarctica lipase B In tetrahydrofuran at 60℃; for 116h; Inert atmosphere; Enzymatic reaction; regioselective reaction; | 93% |
-
-
50-89-5
thymidine

-
-
72072-06-1
5-((tert-butoxycarbonyl)amino)-4-oxopentanoic acid

-
-
1131245-46-9
3',5'-di-O-(Boc-ALA)thymidine

| Conditions | Yield |
|---|---|
| With dmap; triethylamine; dicyclohexyl-carbodiimide In 1,4-dioxane; dichloromethane at 0 - 20℃; | 98% |
-
-
69304-37-6
1,3-Dichloro-1,1,3,3-tetraisopropyldisiloxane

-
-
50-89-5
thymidine

| Conditions | Yield |
|---|---|
| In pyridine at 20℃; for 24h; | 98% |
-
-
69304-37-6
1,3-Dichloro-1,1,3,3-tetraisopropyldisiloxane

-
-
50-89-5
thymidine

-
-
137174-38-0
1-[(6aS,8R,9aR)-2,2,4,4-tetraisopropyl-6a,8,9,9a-tetrahydro-6H-furo[3,2-f][1,3,5,2,4]trioxadisilocin-8-yl]-5-methylpyrimidine-2,4-dione

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 24h; | 98% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; for 21.5h; | 97.1% |
| In pyridine at 20℃; for 48h; | 92% |
| With pyridine at 100℃; for 0.5h; | 91% |
-
-
40615-36-9
4,4'-dimethoxytrityl chloride

-
-
50-89-5
thymidine

-
-
40615-39-2
5'-O-(4-4'-dimethoxytrityl)thymidine

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 20℃; for 5h; | 97% |
| With pyridine; dmap at 20℃; for 24h; | 96% |
| With pyridine at 20℃; for 12h; | 96% |

| Conditions | Yield |
|---|---|
| With pyridine for 3h; Ambient temperature; | 97% |
| With pyridine at 20℃; for 1h; | 96% |
| at 0 - 55℃; | |
| With dmap In dichloromethane |
-
-
1571-13-7
3,4,5,6-tetrachlorophthalimide

-
-
50-89-5
thymidine

-
-
210490-66-7
4,5,6,7-Tetrachloro-2-[(2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-tetrahydro-furan-2-ylmethyl]-isoindole-1,3-dione

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran Mitsunobu reaction; | 97% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran | 97% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran | 97% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran for 0.25h; |
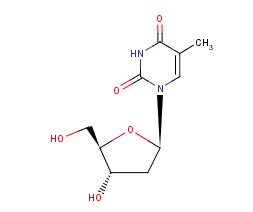
Thymidine Chemical Properties
Molecular structure of Thymidine (CAS NO.50-89-5) is:
Product Name: Thymidine
CAS Registry Number: 50-89-5
IUPAC Name: 1-[(2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)oxolan-2-yl]-5-methylpyrimidine-2,4-dione
Molecular Weight: 242.22856 [g/mol]
Molecular Formula: C10H14N2O5
XLogP3: -1.2
H-Bond Donor: 3
H-Bond Acceptor: 5
Melting Point: 186-188 °C(lit.)
Refractive index: 33 ° (C=1, 1mol/L NaOH)
Storage temp.: 0-6°C
Water Solubility: Soluble
Stability: Stable. Incompatible with strong oxidizing agents.
Surface Tension: 62.2 dyne/cm
Density: 1.452 g/cm3
Product Categories: Fine Chemical & Intermediates; Pyridines, Pyrimidines, Purines and Pteredines; chiral; API intermediates; Zidovudine; Stavudine; Biochemistry; Nucleosides and their analogs; Nucleosides, Nucleotides & Related Reagents; Anti-virals; Bases & Related Reagents; Carbohydrates & Derivatives; Intermediates & Fine Chemicals; Nucleotides; Pharmaceuticals; Miscellaneous Specialties
Thymidine Uses
In cell biology, Thymidine (CAS NO.50-89-5) is used to synchronize the cells in S phase. It is commonly used in cell proliferation assays. It is used as pharmaceutical intermediates, it is used for synthesis of antiviral and anti-HIV drugs.
Thymidine Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 2512mg/kg (2512mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) | Journal of Pharmacology and Experimental Therapeutics. Vol. 207, Pg. 504, 1978. |
Thymidine Consensus Reports
Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
Thymidine Safety Profile
Safty information about Thymidine (CAS NO.50-89-5) is:
Moderately toxic by intraperitoneal route. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits toxic fumes of NOx.
Hazard Codes:  Xi
Xi
Risk Statements: 20/21/22-40-36/37/38
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed
R40: Limited evidence of a carcinogenic effect
R36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements 22-24/25-37/39-26
S22: Do not breathe dust
S24/25: Avoid contact with skin and eyes
S37/39: Wear suitable gloves and eye/face protection
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
F 10: Keep under argon.
WGK Germany: 3
RTECS: XP2071000
Thymidine Specification
Thymidine , its cas register number is 50-89-5. It also can be called 1-(2-Deoxy-beta-D-erythro-pentofuranosyl)-5-methyl-2,4(1H,3H)-pyrimidinedione ; 2'-Deoxythymidine ; 2,4(1H,3H)-Pyrimidinedione, 1-(2-deoxy-beta-D-erythro-pentofuranosyl)-5-methyl- ; 5-Methyl-2'-deoxyuridine ; 5-Methyldeoxyuridine ; 5-Methyldeoxyurindine ; AI3-52267 ; CCRIS 1283 ; DThyd ; Deoxythymidine ; NSC 21548 ; Thymidin ; Thymidine ; Thymine-2-deoxyriboside ; Thymine-2-desoxyriboside ; Thyminedeoxyriboside ; UNII-VC2W18DGKR ; Uridine, 2'-deoxy-5-methyl-beta-D-Ribofuranoside, thymine-1 2-deoxy- ; dT . It is a white crystalline powder.
Related Products
- Thymidine
- Thymidine 5'-(tetrahydrogen triphosphate)
- Thymidine, 2,3-didehydro-3-deoxy-5-O-(8-methoxy-2-oxido-4H-1,3,2-benzodioxaphosphorin-2-yl)-
- Thymidine, 3'-[5-(dimethylamino)-1-naphthalenesulfonate](9CI)
- Thymidine, 3'-acetate5'-[bis(1-aziridinyl)phosphinate] (9CI)
- Thymidine, 3'-acetate5'-[bis(2,2-dimethyl-1-aziridinyl)phosphinate] (9CI)
- Thymidine, 3'-benzoate
- Thymidine, 3-chloro- (9CL)
- Thymidine, 3'-deoxy-,5'-(hydrogen methylphosphonate) (9CI)
- Thymidine, 4'-methoxy-(9CI)
- 50895-58-4
- 50896-54-3
- 50896-90-7
- 5089-70-3
- 5089-72-5
- 50899-10-0
- 50901-18-3
- 50903-76-9
- 50904-38-6
- 50906-05-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View