-
Name
Anethole
- EINECS 203-205-5
- CAS No. 104-46-1
- Article Data69
- CAS DataBase
- Density 0.988 g/mL at 25 °C(lit.)
- Solubility
- Melting Point 20-21 °C(lit.)
- Formula C10H12O
- Boiling Point 237.5 °C at 760 mmHg
- Molecular Weight 148.205
- Flash Point 88.4 °C
- Transport Information
- Appearance Colourless,clear, liquid
- Safety 36/37
- Risk Codes 43
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Anise camphor;Isoestragole;NSC 4018;Nauli gum;Oil of aniseed;p-1-Propenylanisole;p-Anethole;p-Methoxy-b-methylstyrene;p-Propenylanisole;p-Propenylphenyl methyl ether;Anisole,p-propenyl- (8CI);Benzene, 1-methoxy-4-(1-propenyl)- (9CI);1-Methoxy-4-(1-propenyl)benzene;1-Methoxy-4-propenylbenzene;1-Propene,1-(4-methoxyphenyl)-;4-(1-Propenyl)anisole;4-Methoxy-1-propenylbenzene;4-Methoxypropenylbenzene;4-Propenylanisole;Anethol;
- PSA 9.23000
- LogP 2.72830
Synthetic route

| Conditions | Yield |
|---|---|
| With bis(triphenylphosphine)nickel(II) chloride In diethyl ether for 12h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With organo lithium reagent In hexane at 20℃; for 24h; | A 80% B 86% |

| Conditions | Yield |
|---|---|
| With 1-methyl-pyrrolidin-2-one; cobalt acetylacetonate In tetrahydrofuran at 15 - 20℃; for 0.25h; | 80% |

| Conditions | Yield |
|---|---|
| With C68H72Cl2N6NiP2 In tetrahydrofuran at 50℃; for 12h; Inert atmosphere; | 77% |
-
-
7547-96-8, 7547-97-9, 88982-39-2, 6336-44-3
1-propenylboronic acid

-
-
623-12-1
4-chloromethoxybenzene

-
-
104-46-1
anethole

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); caesium carbonate In 1,4-dioxane for 24h; Suzuki-Miyaura cross-coupling; Heating; | 58% |
-
-
74568-53-9
(E)-methyl 3-(3-bromo-4-hydroxyphenyl)acrylate

-
A
-
140-67-0
Estragole

-
B
-
74568-54-0
trans-2-bromo-4-(3-hydroxypropen-1-yl)phenol

-
C
-
104-46-1
anethole

| Conditions | Yield |
|---|---|
| With aluminium hydride In diethyl ether for 1h; Ambient temperature; Yields of byproduct given; | A n/a B 48% C n/a |

-
-
24393-56-4, 51507-22-3, 1929-30-2
ethyl p-methoxycinnamate

-
A
-
140-67-0
Estragole

-
B
-
53484-50-7, 17581-85-0
p-methoxycinnamyl alcohol

-
C
-
104-46-1
anethole

| Conditions | Yield |
|---|---|
| With aluminium hydride In diethyl ether for 0.5h; Ambient temperature; Yields of byproduct given; | A n/a B 47% C n/a |


-
-
104-46-1
anethole

| Conditions | Yield |
|---|---|
| Stage #1: carbonic acid 1-(4-methoxy-phenyl)-allyl ester methyl ester With bis(1,5-cyclooctadiene)diiridium(I) dichloride; dibenzyl ether; N-isopropylidene-N’-2-nitrobenzenesulfonyl hydrazine In acetonitrile for 18h; Sealed tube; Inert atmosphere; Glovebox; Stage #2: With acetic acid In tetrahydrofuran; 2,2,2-trifluoroethanol; water for 2h; Sealed tube; Inert atmosphere; Glovebox; regioselective reaction; | 45% |

| Conditions | Yield |
|---|---|
| With (2,2'-bipyridine)nickel(II) dibromide; tetrabutylammonium tetrafluoroborate In N,N-dimethyl-formamide at 70℃; i = 250 mA, nickel-sponge cathode; | 44% |
-
-
140-67-0
Estragole

-
A
-
87797-12-4
1-methoxy-4-(4-(p-methoxyphenyl)but-3-enyl)benzene

-
B
-
4705-34-4
4,4'-dimethoxystilbene

-
C
-
34414-53-4
1,3-bis(4-methoxyphenyl)prop-1-ene

-
D
-
104-46-1
anethole

| Conditions | Yield |
|---|---|
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In toluene at 40℃; Inert atmosphere; Schlenk technique; | A n/a B n/a C n/a D n/a E 41% |


| Conditions | Yield |
|---|---|
| With trityl tetrafluoroborate In dichloromethane at -78℃; for 30h; | 15% |

| Conditions | Yield |
|---|---|
| With infusorial earht at 500℃; | |
| iridium(III) chloride; β‐cyclodextrin In water at 95℃; for 2.5h; | |
| PEG-KOH In potassium hydroxide; toluene at 65℃; Mechanism; Rate constant; variations of stirring rate, aqueous KOH concentration, and catalyst (7-130 oxyethylene units / PEG monomethyl, dimethyl, and dibenzyl ethers), other temp.; activation energy; |

-
-
108-24-7
acetic anhydride

-
A
-
127-19-5
N,N-dimethyl acetamide

-
B
-
16031-55-3
1-(4-methoxyphenyl)prop-1-yl acetate

-
C
-
104-46-1
anethole


| Conditions | Yield |
|---|---|
| With sodium proprionate |
-
-
187737-37-7
propene

-
-
5780-90-5
(4-Methoxyphenyl)mercuric acetate

-
A
-
140-67-0
Estragole

-
B
-
1712-69-2
1-isopropenyl-4-methoxybenzene

-
C
-
104-46-1
anethole

| Conditions | Yield |
|---|---|
| (i) Pd(OAc)2, MeCN, (ii) /BRN= 1696878/; Multistep reaction; |


| Conditions | Yield |
|---|---|
| With H2O2 or O2; copper dichloride; palladium dichloride In ethanol |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide; hexane Yield given; | |
| Stage #1: ethyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 0℃; for 0.5h; Stage #2: 4-methoxy-benzaldehyde In tetrahydrofuran at 20℃; for 12h; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 1h; | 52 % Chromat. |

-
A
-
1712-69-2
1-isopropenyl-4-methoxybenzene

-
B
-
4030-17-5
4-methoxyphenylcyclopropane

-
C
-
104-46-1
anethole

| Conditions | Yield |
|---|---|
| at 160℃; Yield given. Yields of byproduct given; |


-
-
104-46-1
anethole

-
-
104-46-1
anethole

| Conditions | Yield |
|---|---|
| With sulfuric acid durch Destillation; |

-
-
104-46-1
anethole

| Conditions | Yield |
|---|---|
| With potassium carbonate | |
| With pyridine |

-
-
104-46-1
anethole

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| at 500℃; |
-
-
16031-55-3
1-(4-methoxyphenyl)prop-1-yl acetate

-
-
104-46-1
anethole

| Conditions | Yield |
|---|---|
| With C28H18Co(1-)*K(1+)*2C4H10O2; hydrogen In toluene at 60℃; under 7500.75 Torr; for 24h; chemoselective reaction; | 99% |
| With sodium hydrogen telluride In ethanol Heating; overnight; | 94% |
| With formic acid; Pd(SIPr)(PCy3) In tetrahydrofuran at 60℃; for 24h; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| With iodic acid; potassium bromide In dichloromethane; water at 20℃; for 0.166667h; | 99% |
| With periodic acid; potassium bromide In dichloromethane; water at 20℃; for 0.133333h; | 98% |
| With diethyl ether; bromine |
-
-
89845-28-3
2-(2-phenylethynyl)-1,4-benzoquinone

-
-
104-46-1
anethole

-
-
1432504-68-1
2-(4-methoxyphenyl)-2-methyl-7-(phenylethynyl)-2,3-dihydrobenzofuran-5-ol

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In acetonitrile at 0℃; Inert atmosphere; Schlenk technique; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: propionic acid With 2,6-dimethylpyridine; 10-methyl-9-(2,4,6-trimethylphenyl) acridinium tetrafluoroborate; thiophenol In 1,2-dichloro-ethane at 0 - 23℃; for 0.25h; Inert atmosphere; Stage #2: anethole In 1,2-dichloro-ethane for 30h; Inert atmosphere; Irradiation; regioselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: isobutyric Acid With 2,6-dimethylpyridine; 10-methyl-9-(2,4,6-trimethylphenyl) acridinium tetrafluoroborate; thiophenol In 1,2-dichloro-ethane at 0 - 23℃; for 0.25h; Inert atmosphere; Stage #2: anethole In 1,2-dichloro-ethane for 62h; Inert atmosphere; Irradiation; regioselective reaction; | 99% |
-
-
887144-94-7
Togni's reagent II

-
-
104-46-1
anethole

-
-
1610846-52-0
3,3,3-trifluoro-1-(4-methoxyphenyl)-2-methylpropan-1-one

| Conditions | Yield |
|---|---|
| With tris[2-phenylpyridinato-C2,N]iridium(III); dimethyl sulfoxide at 20℃; for 2h; Irradiation; Green chemistry; | 99% |
| With N9,N9,N10,N10-tetrakis(4-(tert-butyl)phenyl)anthracene-9,10-diamine; dimethyl sulfoxide In dichloromethane at 20℃; for 3h; Irradiation; | 75% |
| With dmap; tris(2,2-bipyridine)ruthenium(II) hexafluorophosphate; magnesium sulfate In dimethyl sulfoxide at 20℃; for 48h; Solvent; Reagent/catalyst; Inert atmosphere; Irradiation; | 40% |
-
-
104-46-1
anethole

-
-
78-79-5
isoprene

-
-
112150-17-1
(1SR,2SR)-4'-methoxy-2,4-dimethyl-1,2,3,6-tetrahydro-1,1'-biphenyl

| Conditions | Yield |
|---|---|
| With N,N’,N’’-tris(3,5-bis(trifluoromethyl)phenyl)thiophosphoramide; [Ru(4,4'-bis(trifluoromethyl)-2,2'-bipyridine)3](3,5-bis(trifluoromethyl)benzenesulfonate)2 In dichloromethane at 20℃; for 0.333333h; Quantum yield; Reagent/catalyst; Diels-Alder Cycloaddition; Inert atmosphere; Irradiation; | 99% |
| With tris(4-bromophenyl)ammoniumyl hexafluoroantimonate In dichloromethane for 1h; Quantum yield; Reagent/catalyst; Diels-Alder Cycloaddition; Irradiation; | 35% |

| Conditions | Yield |
|---|---|
| With copper(ll) bromide In acetonitrile at 20℃; for 2h; Schlenk technique; Inert atmosphere; | 99% |

| Conditions | Yield |
|---|---|
| With copper(ll) bromide In acetonitrile at 20℃; for 2h; Schlenk technique; Inert atmosphere; | 99% |
-
-
104-46-1
anethole

-
-
51410-46-9
2-(4-methoxyphenyl)-3-methyloxirane

| Conditions | Yield |
|---|---|
| With 3,3-dimethyldioxirane In acetone for 0.666667h; Ambient temperature; | 98% |
| With dihydrogen peroxide; sodium hydrogencarbonate In acetonitrile | |
| With oxidovanadium(IV)-β-octabromo-meso-tetrakis(2,6-dibromo-3,5-dimethoxyphenyl)porphyrin; dihydrogen peroxide; sodium hydrogencarbonate In water; acetonitrile at 60℃; for 0.5h; Catalytic behavior; Reagent/catalyst; |

| Conditions | Yield |
|---|---|
| With tris(1,10-phenanthroline)iron(III) tris(hexafluorophosphate) In 1,2-dichloro-ethane at 0℃; for 6h; | 98% |

| Conditions | Yield |
|---|---|
| Lewatit SP 120 (acid form) In 1,4-dioxane at 60℃; for 0.5h; | 97% |
| With sulfuric acid |
-
-
935-50-2
4,4-dimethoxycyclohexa-2,5-dienone

-
-
104-46-1
anethole

-
-
1326704-90-8
2,3-dihydro-5-methoxy-2-(4-methoxyphenyl)-3-methylbenzofuran

| Conditions | Yield |
|---|---|
| With polystyrene-supported perfluorobenzoic acid In dichloromethane; 1,1,1,3',3',3'-hexafluoro-propanol at 0℃; chemoselective reaction; | 97% |
| With 1,1,1,3',3',3'-hexafluoro-propanol; Pentafluorobenzoic acid In dichloromethane at 25℃; for 0.166667h; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: butyric acid With 2,6-dimethylpyridine; 10-methyl-9-(2,4,6-trimethylphenyl) acridinium tetrafluoroborate; thiophenol In 1,2-dichloro-ethane at 0 - 23℃; for 0.25h; Inert atmosphere; Stage #2: anethole In 1,2-dichloro-ethane for 48h; Inert atmosphere; Irradiation; regioselective reaction; | 97% |

| Conditions | Yield |
|---|---|
| With copper(ll) bromide In acetonitrile at 20℃; for 2h; Solvent; Schlenk technique; Inert atmosphere; | 97% |
-
-
104-46-1
anethole

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

-
-
1589553-46-7
(S)-2-(1-(4-methoxyphenyl)propyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With C24H31Cl2FeN3O2; sodium triethylborohydride In tetrahydrofuran at 20℃; for 1h; Inert atmosphere; | 97% |
| With C26H28N4O; cobalt(II) acetate In diethyl ether at 20℃; for 18h; enantioselective reaction; | 87% |
-
-
1447990-65-9
C12H12O2

-
-
104-46-1
anethole

-
-
1432504-78-3
7-(3,3-dimethylbut-1-ynyl)-2-(4-methoxyphenyl)-2-methyl-2,3-dihydrobenzofuran-5-ol

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In acetonitrile at 0℃; Inert atmosphere; Schlenk technique; | 96% |

| Conditions | Yield |
|---|---|
| With C18H22N2O; copper(II) bis(trifluoromethanesulfonate) In toluene at -80℃; for 36h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 96% |
-
-
104-46-1
anethole

-
-
252058-33-6, 305820-74-0
2-(4-methoxyphenyl)propanal

| Conditions | Yield |
|---|---|
| With iodine; silver(l) oxide In 1,4-dioxane; water for 4h; Ambient temperature; | 95% |
| With N-iodo-succinimide; water; cetyltrimethylammonim bromide In 1,4-dioxane at 20 - 105℃; for 0.333333h; Microwave irradiation; | 78% |
| With N-Bromosuccinimide; water; cetyltrimethylammonim bromide In dimethyl sulfoxide at 200℃; for 0.2h; Microwave irradiation; | 65% |
-
-
104-46-1
anethole

-
A
-
51410-46-9
2-(4-methoxyphenyl)-3-methyloxirane

-
B
-
252058-33-6, 305820-74-0
2-(4-methoxyphenyl)propanal

| Conditions | Yield |
|---|---|
| With iodine; silver(l) oxide In 1,4-dioxane; water for 4h; Product distribution; Ambient temperature; various bases, solvents and temp.; | A n/a B 95% |
| With sodium hydroxide; iodine for 2h; Ambient temperature; | A 10 % Chromat. B n/a |
-
-
104-46-1
anethole

-
-
4705-34-4, 15638-14-9, 34480-01-8, 112246-67-0, 2510-75-0
1,2-bis(4-methoxyphenyl)ethene

| Conditions | Yield |
|---|---|
| With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride In neat (no solvent) at 90℃; for 1.33333h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With copper(ll) bromide In acetonitrile at 20℃; for 2h; Schlenk technique; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With C18H22N2O; copper(II) bis(trifluoromethanesulfonate) In toluene at -80℃; for 36h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With C18H22N2O; copper(II) bis(trifluoromethanesulfonate) In toluene at -80℃; for 36h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With aluminum oxide; potassium permanganate In dichloromethane at 20℃; for 4h; | 94% |
| With disodium hydrogenphosphate; oxygen; cobalt(II) acetate In methanol at 50℃; for 6h; | 89.9% |
| With oxygen; ozone In water; ethyl acetate at 20℃; for 1.66667h; | 81.7% |

| Conditions | Yield |
|---|---|
| Stage #1: benzoic acid With 2,6-dimethylpyridine; 10-methyl-9-(2,4,6-trimethylphenyl) acridinium tetrafluoroborate; thiophenol In 1,2-dichloro-ethane at 0 - 23℃; for 0.25h; Inert atmosphere; Stage #2: anethole In 1,2-dichloro-ethane for 30h; Inert atmosphere; Irradiation; regioselective reaction; | 94% |

| Conditions | Yield |
|---|---|
| With trimethylsilylazide; water; dimethylglyoxal In toluene at 20℃; for 8h; Inert atmosphere; Schlenk technique; | 94% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; tetra-(n-butyl)ammonium iodide In water; acetonitrile at 100℃; for 2h; | 93% |

| Conditions | Yield |
|---|---|
| With C18H22N2O; copper(II) bis(trifluoromethanesulfonate) In toluene at -80℃; for 36h; Schlenk technique; Inert atmosphere; enantioselective reaction; | 93% |
-
-
104-46-1
anethole

-
-
21086-33-9
2-bromo-1-(4-methoxyphenyl)-1-propanone

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione In dimethyl sulfoxide at 25 - 35℃; for 0.3h; regioselective reaction; | 92% |
| With dipotassium peroxodisulfate; potassium bromide In water at 60℃; for 12h; Green chemistry; | 21% |
| With bromine; ethyl acetate for 2h; | 3.9% |
| Multi-step reaction with 2 steps 1: absolute diethyl ether; bromine 2: CrO3; glacial acetic acid View Scheme |
cis-Anethol Chemical Properties
The Molecular formula of p-PROPENYLANISOLE(104-46-1):C10H12O
The Molecular Weight of p-PROPENYLANISOLE(104-46-1):148.2
The Molecular Structure of p-PROPENYLANISOLE(104-46-1) is: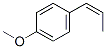
Melting point:20-21 °C(lit.)
Boiling point:237.5 °C at 760 mmHg
Flash point:88.4 °C
Solubility in water:slightly water soluble .
Index of Refraction:1.545
Molar Refractivity:48.82 cm3
Molar Volume:154.4 cm3
Polarizability:19.35 10-24cm3
Surface Tension:31.8 dyne/cm
Enthalpy of Vaporization:45.51 kJ/mol
Vapour Pressure:0.0687 mmHg at 25°C
Appearance:white crystals or a liquid
IUPAC Name:1-methoxy-4-[(E)-prop-1-enyl]benzene
Synonyms:P-METHOXYPROPENYLBENZENE;P-PROPENYLPHENYL METHYL ETHER;TRANS-1-METHOXY-4-(1-PROPENYL)BENZENE;TRANS-P-METHOXYPROPENYLBENZENE;TRANS-P-PROPENYLANISOLE;FEMA 2086;(E)-1-METHOXY-4-(1-PROPENYL)BENZENE;PARA METHOXY ALPHA PHENYL PROPENE
cis-Anethol Uses
cis-Anethol Toxicity Data With Reference
| 1. | orl-rat LD50:2090 mg/kg | FCTXAV Food and Cosmetics Toxicology. 2 (1964),327. | ||
| 2. | orl-mus LD50:3050 mg/kg | FCTXAV Food and Cosmetics Toxicology. 2 (1964),327. | ||
| 3. | orl-gpg LD50:2160 mg/kg | FCTXAV Food and Cosmetics Toxicology. 2 (1964),327. | ||
| 4. | orl-bwd LD50:316 mg/kg | AECTCV Archives of Environmental Contamination and Toxicology. 12 (1983),355. |
cis-Anethol Consensus Reports
cis-Anethol Safety Profile
Related Products
- cis-Anethol
- 104465-34-1
- 1044664-24-5
- 10447-11-7
- 104-47-2
- 104472-68-6
- 1044764-16-0
- 1044767-99-8
- 1044768-40-2
- 104484-71-1
- 10448-49-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View