-
Name
1,3,5-trioxane
- EINECS 203-812-5
- CAS No. 110-88-3
- Article Data46
- CAS DataBase
- Density 1.131 g/cm3
- Solubility 221 g/L (25 °C) in water
- Melting Point 59-62 °C(lit.)
- Formula C3H6O3
- Boiling Point 114.5 °C at 760 mmHg
- Molecular Weight 90.0788
- Flash Point 45 °C
- Transport Information UN 1325 4.1/PG 2
- Appearance colourless transparent crystals
- Safety 36/37-46
- Risk Codes 11-37-63
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xn
Xn
- Synonyms Trioxane(6CI,7CI);1,3,5-Trioxane;Formaldehyde, trimer;NSC 26347;Triformol;Trioxan;Trioxymethylene;sym-Trioxane;
- PSA 27.69000
- LogP -0.07770
Synthetic route
-
-
1670-46-8
2-acetylcyclopentanaone

-
A
-
110-88-3
1,3,5-Trioxan

-
B
-
96-48-0
4-butanolide

-
C
-
108-55-4
glutaric anhydride,

-
D
-
64-18-6
formic acid

-
E
-
110-94-1
1,5-pentanedioic acid

-
F
-
123-42-2
4-Hydroxy-4-methyl-2-pentanone

-
G
-
26976-72-7
4-acetoxybutyric acid

-
H
-
85951-55-9
5,6-dioxo-n-heptanoic acid

-
I
-
1262892-77-2
2-acetyl-2-hydroxycyclopentanone

-
J
-
52789-75-0
2-acetoxycyclopentanone

-
K
-
1167443-17-5
2-acetyl-2-hydroxymethylcyclopentanone

-
L
-
1426960-55-5
1,1'-diacetyl-1,1'-bicyclopentyl-2,2'-dione

-
M
-
89540-15-8
2-acetyl-2,3-epoxycyclopentanone

-
N
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With oxygen; calcium chloride In acetone at 57℃; for 30h; | A 1% B 2% C 7% D n/a E 7% F 0.014 g G 2% H 13% I 38% J 2% K 5% L 11% M 2% N n/a |

-
-
1670-46-8
2-acetylcyclopentanaone

-
A
-
110-88-3
1,3,5-Trioxan

-
B
-
96-48-0
4-butanolide

-
C
-
108-55-4
glutaric anhydride,

-
D
-
110-94-1
1,5-pentanedioic acid

-
E
-
1262892-77-2
2-acetyl-2-hydroxycyclopentanone

-
F
-
52789-75-0
2-acetoxycyclopentanone

-
G
-
1167443-17-5
2-acetyl-2-hydroxymethylcyclopentanone

-
H
-
89540-15-8
2-acetyl-2,3-epoxycyclopentanone

| Conditions | Yield |
|---|---|
| With oxygen In acetone at 20℃; for 4h; Irradiation; | A 1% B 3% C 3% D 13% E 36% F 1% G 22% H 2% |

-
-
1670-46-8
2-acetylcyclopentanaone

-
A
-
110-88-3
1,3,5-Trioxan

-
B
-
96-48-0
4-butanolide

-
C
-
108-55-4
glutaric anhydride,

-
D
-
64-18-6
formic acid

-
E
-
110-94-1
1,5-pentanedioic acid

-
F
-
1262892-77-2
2-acetyl-2-hydroxycyclopentanone

-
G
-
52789-75-0
2-acetoxycyclopentanone

-
H
-
1167443-17-5
2-acetyl-2-hydroxymethylcyclopentanone

-
I
-
89540-15-8
2-acetyl-2,3-epoxycyclopentanone

-
J
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With oxygen In acetone at 20℃; for 6h; Irradiation; | A 2% B 4% C 5% D n/a E 17% F 18% G 2% H 30% I 3% J n/a |

-
-
93-58-3
benzoic acid methyl ester

-
A
-
110-88-3
1,3,5-Trioxan

-
B
-
92-52-4
biphenyl

-
C
-
16606-00-1
methyl 3-phenylbenzoate

-
D
-
71-43-2
benzene

| Conditions | Yield |
|---|---|
| thermische Zersetzung an einem hellrotgluehenden Draht.Thermolysis; |


| Conditions | Yield |
|---|---|
| With water at 100℃; im Vakuum; | |
| With sulfuric acid at 115℃; im evakuierten Rohr; | |
| With sulfuric acid Destillieren des Reaktionsprodukts mit Kohlendioxyd in Eiswasser; |

| Conditions | Yield |
|---|---|
| With phosphotungstic acid; water at 96.5℃; Kinetics; Product distribution; other heteropolyacids; | |
| C15H16N2O4(2+)*HO4P(2-) In methanol; water at 92 - 97℃; for 4h; Product distribution / selectivity; |

| Conditions | Yield |
|---|---|
| With [Ru3Ir(CO)13](1-)*[N(PPh3)2](1+) at 90℃; for 48h; Dehydrogenation; trimerization; |

-
-
110-88-3
1,3,5-Trioxan

| Conditions | Yield |
|---|---|
| With water at 100℃; im Vakuum; |

| Conditions | Yield |
|---|---|
| With water at 100℃; im Vakuum; |

-
-
110-88-3
1,3,5-Trioxan

| Conditions | Yield |
|---|---|
| With nitrogen |

-
-
110-88-3
1,3,5-Trioxan

| Conditions | Yield |
|---|---|
| With sulfuric acid at 115℃; im Rohr; |

| Conditions | Yield |
|---|---|
| at 75℃; Oxydation; |

-
-
4382-76-7
methoxymethyl acetate

-
-
7732-18-5
water

-
A
-
110-88-3
1,3,5-Trioxan

-
B
-
67-56-1
methanol

-
C
-
64-19-7
acetic acid


-
-
80653-57-2
N,O-Dimesyl-N-methylhydroxylamin

-
A
-
110-88-3
1,3,5-Trioxan

-
B
-
50-00-0
formaldehyd

-
C
-
593-77-1
N-Methylhydroxylamine

-
D
-
4229-44-1
N-methylhydroxyamine hydrochloride

-
E
-
2386-57-4
methanesulfonic acid sodium salt

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water Mechanism; Product distribution; hydrolysis with basic, neutral and acidic aqu. solutions; | A 5 % Spectr. B n/a C 1 % Spectr. D n/a E 33 % Spectr. F n/a |

-
-
50-00-0
formaldehyd

-
A
-
110-88-3
1,3,5-Trioxan

-
B
-
67-56-1
methanol

-
C
-
534-15-6
1,1-dimethoxyethane

-
D
-
107-31-3
Methyl formate

| Conditions | Yield |
|---|---|
| With water under 75.0075 - 7500.75 Torr; Gas phase; Acidic conditions; |


| Conditions | Yield |
|---|---|
| silicotungstic acid In water at 100℃; Product distribution; Further Variations:; Catalysts; Temperatures; Cyclization; Cannizzaro reaction; Etherification; |


| Conditions | Yield |
|---|---|
| C7H7O3S(1-)*C10H19O3S(1+) In methanol; water at 92 - 96.5℃; for 3h; Product distribution / selectivity; Heating / reflux; | |
| 1-(4-sulfonylbutyl)pyridinium trifluoromethanesulfonate In methanol; water at 92 - 97℃; for 3h; Product distribution / selectivity; Heating / reflux; | |
| CF3O3S(1-)*C8H12NO3S(1+) In methanol; water at 93 - 97℃; for 3h; Product distribution / selectivity; Heating / reflux; |

| Conditions | Yield |
|---|---|
| CF3O3S(1-)*C10H19O3S(1+) In methanol; water at 94 - 96.5℃; for 4h; Product distribution / selectivity; Heating / reflux; |


| Conditions | Yield |
|---|---|
| 2CF3O3S(1-)*C11H18N4O3S(2+) In water at 92 - 97℃; for 7h; Product distribution / selectivity; Heating / reflux; | |
| 1-butyl-3-methylimidazolium hydrogen sulfate In water at 92 - 97℃; for 7h; Product distribution / selectivity; Heating / reflux; | |
| tetrabutylammonium bis[(trifluoromethane)sulfonyl]imide In water at 93 - 97℃; for 7h; Product distribution / selectivity; Heating / reflux; |

-
-
67-56-1
methanol

-
-
50-00-0
formaldehyd

-
A
-
110-88-3
1,3,5-Trioxan

-
B
-
64-18-6
formic acid

-
C
-
109-87-5
Dimethoxymethane

| Conditions | Yield |
|---|---|
| 2C7H7O3S(1-)*C15H26N4O6S2(2+) In water at 94 - 96.5℃; for 4h; Product distribution / selectivity; |

-
-
50-00-0
formaldehyd

-
-
109-87-5
Dimethoxymethane

-
A
-
110-88-3
1,3,5-Trioxan

-
D
-
13353-03-2
bis-methoxymethoxy-methane

-
E
-
628-90-0
bis(methoxymethyl)ether

-
F
-
13352-75-5
POMM4

-
G
-
13352-78-8
CH3-(OCH2)8-OCH3

-
H
-
54261-86-8
Methoxymethoxymethoxymethoxymethoxy-methoxymethoxymethoxymethoxymethoxymethoxy-methane

-
I
-
13353-04-3
CH3-(OCH2)7-OCH3

-
L
-
13352-77-7
CH3-(OCH2)6-OCH3

| Conditions | Yield |
|---|---|
| With sulfuric acid at 55 - 115℃; Inert atmosphere; |

-
-
64-17-5
ethanol

-
A
-
110-88-3
1,3,5-Trioxan

-
B
-
105-57-7
diethyl acetal

-
C
-
60-29-7
diethyl ether

-
D
-
75-07-0
acetaldehyde

| Conditions | Yield |
|---|---|
| With silicon carbide at 250℃; under 750.075 Torr; for 3h; Temperature; |


| Conditions | Yield |
|---|---|
| With silicon carbide at 225℃; under 750.075 Torr; for 3h; |

-
-
64-17-5
ethanol

-
A
-
110-88-3
1,3,5-Trioxan

-
B
-
105-57-7
diethyl acetal

-
C
-
75-07-0
acetaldehyde

-
D
-
64-19-7
acetic acid

| Conditions | Yield |
|---|---|
| With silicon carbide; hydrotalcite at 150℃; under 750.075 Torr; for 3h; |


| Conditions | Yield |
|---|---|
| With silicon carbide at 150℃; under 750.075 Torr; for 3h; |

| Conditions | Yield |
|---|---|
| With silicon carbide at 150℃; under 750.075 Torr; for 3h; |


| Conditions | Yield |
|---|---|
| With silicon carbide at 200℃; under 750.075 Torr; for 3h; |
-
-
110-88-3
1,3,5-Trioxan

-
-
19299-41-3
N-methyl-1-phenylmethanesulfonamide

-
-
61199-72-2
3-methyl-3,4-dihydro-1H-benzo[d][1,2]thiazine 2,2-dioxide

| Conditions | Yield |
|---|---|
| With Amberlyst 15 In 1,2-dichloro-ethane at 35℃; for 3h; | 100% |
| With methanesulfonic acid; trifluoroacetic acid at 35℃; for 0.5h; | 78% |
| With silica-supported molybdophosphoric heteropolyacid In toluene at 110℃; for 4.5h; | 68% |
| With methanesulfonic acid In trifluoroacetic acid |
-
-
110-88-3
1,3,5-Trioxan

-
-
4563-33-1
phenyl-methanesulfonic acid amide

-
-
33183-87-8
3,4-dihydro-1H-benzo[d][1,2]thiazine 2,2-dioxide

| Conditions | Yield |
|---|---|
| With Amberlyst 15 In 1,2-dichloro-ethane at 80℃; for 3h; | 100% |
| With sulfated zirconia at 115℃; for 6h; | 82% |
| With amberlyst 15H+ resin In 1,2-dichloro-ethane at 80℃; | 79% |
| With methanesulfonic acid; acetic anhydride In 1,2-dichloro-ethane at 35℃; for 4h; | 68% |
| With silica-supported molybdophosphoric heteropolyacid In toluene at 110℃; for 4.5h; | 62% |
-
-
110-88-3
1,3,5-Trioxan

-
-
85952-14-3
N-ethyl-benzylsulfonamide

-
-
33050-15-6
3-ethyl-3,4-dihydro-1H-benzo[d][1,2]thiazine 2,2-dioxide

| Conditions | Yield |
|---|---|
| With Amberlyst 15 In 1,2-dichloro-ethane at 35℃; for 3h; | 100% |
| With sulfated zirconia at 115℃; for 3h; | 96% |
| With methanesulfonic acid; trifluoroacetic acid at 35℃; for 0.5h; | 88% |
| With silica-supported molybdophosphoric heteropolyacid In toluene at 110℃; for 4.5h; | 88% |
-
-
110-88-3
1,3,5-Trioxan

-
-
85952-15-4
N-isopropyl-1-phenyl methanesulfonamide

-
-
110654-43-8
3-Isopropyl-3,4-dihydro-1H-benzo[d][1,2]thiazine 2,2-dioxide

| Conditions | Yield |
|---|---|
| With Amberlyst 15 In 1,2-dichloro-ethane at 35℃; for 3h; | 100% |
| With methanesulfonic acid; trifluoroacetic acid at 35℃; for 0.5h; | 90% |
| With silica-supported molybdophosphoric heteropolyacid In toluene at 110℃; for 4.5h; | 87% |
| With sulfated zirconia at 115℃; for 6h; | 57% |
-
-
110-88-3
1,3,5-Trioxan

-
-
64-19-7
acetic acid

-
-
153528-91-7
4,5-dimethoxy-N-(3-hydroxypropyl) salicylamide

-
-
153528-89-3
2,3-dihydro-6,7-dimethoxy-3-(3-acetoxypropyl)-4H-1,3-benzoxazin-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethyl acetate for 16h; Ambient temperature; | 100% |

-
-
110-88-3
1,3,5-Trioxan

-
-
878672-60-7
tert-butyl 2-(5-bromo-2-nitrophenyl)ethanoate

-
-
1033265-31-4
tert-butyl 2-(5-bromo-2-nitrophenyl)propenoate

| Conditions | Yield |
|---|---|
| With piperidine; acetic acid In benzene for 48h; Knoevenagel-type condensation; Heating; | 100% |
-
-
110-88-3
1,3,5-Trioxan

-
-
81327-28-8
tert-butyl 2-(5-chloro-2-nitrophenyl)ethanoate

-
-
1033265-33-6
tert-butyl 2-(5-chloro-2-nitrophenyl)propenoate

| Conditions | Yield |
|---|---|
| With piperidine; acetic acid In benzene for 120h; Knoevenagel-type condensation; Heating; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-Trioxan; C17H27ClO2Si With zirconium(IV) chloride In dichloromethane for 2h; Inert atmosphere; Stage #2: With sodium hydrogencarbonate In dichloromethane | 100% |
-
-
110-88-3
1,3,5-Trioxan

| Conditions | Yield |
|---|---|
| With triethylsilane; trifluoroacetic acid In dichloromethane at 20℃; for 48h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| With perchloric acid at 65 - 110℃; Inert atmosphere; | 99.7% |

| Conditions | Yield |
|---|---|
| With trimethylsilyl iodide at 40℃; for 10h; | A 99.5% B 98% |
-
-
110-88-3
1,3,5-Trioxan

-
-
2388-68-3
o-bis(mercaptomethyl)benzene

-
-
7216-19-5
1,5-dihydrobenzo[e]-1,3-dithiepine

| Conditions | Yield |
|---|---|
| With Montmorillonite KSF In benzene for 1.5h; Heating; | 99% |
-
-
110-88-3
1,3,5-Trioxan

-
-
93624-87-4
α-p-methoxy phenoxymethyl β-N-carbomethoxy aminoxyethanol

-
-
93625-07-1
carbomethoxy-3 p-methoxy phenoxymethyl-5 tetrahydrodioxazine-1,4,2

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 4h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid for 2h; Heating; | 99% |
| With hydrogen bromide; acetic acid for 3h; Reflux; | 95% |
| With hydrogen bromide; acetic acid for 2h; Heating; | 90% |
| With hydrogen bromide In acetic acid |
-
-
110-88-3
1,3,5-Trioxan

-
-
24623-65-2
3-tert-butyl-2-hydroxybenzaldehyde

-
-
183017-88-1
3-tert-butyl-5-(chloromethyl)-2-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 40℃; for 72h; | 99% |
| With hydrogenchloride In water at 60℃; for 72h; | 97% |
| With hydrogenchloride In water at 40℃; for 72h; | 90% |
| With hydrogenchloride In water at 45 - 50℃; for 48h; | 90% |
-
-
110-88-3
1,3,5-Trioxan

-
-
1101186-75-7
C15H19NO2S2

| Conditions | Yield |
|---|---|
| With titanium tetrachloride; triethylamine optical yield given as %de; | 99% |
| Stage #1: 1-[(4S)-4-benzyl-2-thioxo(1,3-thiazolidin-3-yl)]butan-1-one With titanium tetrachloride In dichloromethane at 0℃; for 0.166667h; Inert atmosphere; Stage #2: With triethylamine In dichloromethane at 0℃; for 0.55h; Stage #3: 1,3,5-Trioxan With titanium tetrachloride In dichloromethane at 0℃; for 2h; optical yield given as %de; diastereoselective reaction; | 81% |

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid for 2.5h; Inert atmosphere; Reflux; | 99% |

| Conditions | Yield |
|---|---|
| With triethylsilane; trifluoroacetic acid In dichloromethane at 20℃; for 48h; Inert atmosphere; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With triethylsilane; trifluoroacetic acid In dichloromethane at 20℃; for 24h; Inert atmosphere; chemoselective reaction; | 99% |

| Conditions | Yield |
|---|---|
| With phenylsulfonic acid functionalized mesoporous silica In 1,2-dichloro-ethane Reflux; | 98.1% |
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; trifluoroacetic acid at 35℃; for 0.5h; | 98% |

| Conditions | Yield |
|---|---|
| In formic acid aq. formic acid; Ru-contg. compd. (20.5 mmol) and paraformaldehyde were added to a soln. of formic acid; the soln. was heated at reflux for 5 h; then orange soln. was cooled in an ice bath and stored in the freezer overnight (-4°C); the solvent was removed by rotary evapn.; the solid was washed with hexane and dried in vac.; | 98% |
-
-
110-88-3
1,3,5-Trioxan

-
-
69688-96-6
N-butyl-1-phenylmethanesulfonamide

-
-
1254217-19-0
N-butyl-3,4-dihydro-1H-2,3-benzothiazine 2,2-dioxide

| Conditions | Yield |
|---|---|
| With sulfated zirconia at 115℃; for 3h; | 98% |
-
-
110-88-3
1,3,5-Trioxan

| Conditions | Yield |
|---|---|
| With Amberlyst XN-1010 In 1,2-dichloro-ethane at 80℃; for 3h; | 98% |
-
-
110-88-3
1,3,5-Trioxan

-
-
60833-52-5
iodo(iodomethoxy)methane

| Conditions | Yield |
|---|---|
| With trimethylsilyl iodide at 40℃; for 15h; | 98% |
| With trimethylsilyl iodide at 40℃; Darkness; | 88.2% |
-
-
110-88-3
1,3,5-Trioxan

-
-
5279-03-8
2-methyl-6,7-dihydrobenzo[b]thiophene-4(5H)-one

-
-
95526-48-0
2-Methyl-5-methylene-6,7-dihydro-5H-benzo[b]thiophen-4-one

| Conditions | Yield |
|---|---|
| With N-methylanilinium trifluoroacetate In tetrahydrofuran | 97% |
-
-
110-88-3
1,3,5-Trioxan

| Conditions | Yield |
|---|---|
| With Amberlyst XN-1010 In 1,2-dichloro-ethane at 80℃; for 3h; | 97% |
-
-
110-88-3
1,3,5-Trioxan

-
-
34451-26-8
2-perfluorohexylethanethiol

-
-
114857-05-5
(F-hexyl-2 ethylthio) methanol

| Conditions | Yield |
|---|---|
| With triethylamine at 0℃; Mechanism; | 96% |
| With triethylamine at 0℃; | 96% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid In ethyl acetate at 80℃; | 96% |
-
-
110-88-3
1,3,5-Trioxan

| Conditions | Yield |
|---|---|
| With Amberlyst XN-1010 In 1,2-dichloro-ethane at 80℃; for 3h; | 96% |
s-Trioxane Consensus Reports
s-Trioxane Specification
The s-Trioxane with CAS registry number of 110-88-3 is also known as Trioxymethylene. The IUPAC name is 1,3,5-Trioxane. Its EINECS registry number is 203-812-5. In addition, the formula is C3H6O3 and the molecular weight is 90.08. This chemical is a white crystal that may cause damage to health and may catch fire in contact with air, only need brief contact with an ignition source. What's more, it can be used as chemical intermediate and disinfectant that should be sealed in ventilated, cool place away from oxidants, acids.
Physical properties about s-Trioxane are: (1)ACD/LogP: -0.62; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.62; (4)ACD/LogD (pH 7.4): -0.62; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 10.98; (8)ACD/KOC (pH 7.4): 10.98; (9)#H bond acceptors: 3; (10)Index of Refraction: 1.385; (11)Molar Refractivity: 18.65 cm3; (12)Molar Volume: 79.5 cm3; (13)Surface Tension: 35.2 dyne/cm; (14)Density: 1.131 g/cm3; (15)Flash Point: 45 °C; (16)Enthalpy of Vaporization: 33.83 kJ/mol; (17)Boiling Point: 114.5 °C at 760 mmHg; (18)Vapour Pressure: 23.5 mmHg at 25 °C.
Preparation of s-Trioxane. 60 % Formaldehyde solution containing 2 % to 5 % sulfuric acid is distilled continuously. Then the residue is extracted with benzene and distilled to obtain the prodcut.
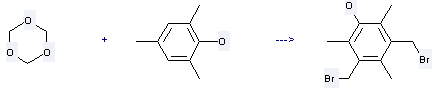
Uses of s-Trioxane: it is used to produce 3,5-bis-bromomethyl-2,4,6-trimethyl-phenol by reaction with 2,4,6-trimethyl-phenol. The reaction occurs with reagents HBr, AcOH and other condition of heating. The yield is about 99 %.
When you are using this chemical, please be cautious about it. As a chemical, it is irritating to respiratory system and highly flammable. There is possible risk of harm to the unborn child. During using it, wear suitable protective clothing and gloves. If swallowed, seek medical advice immediately and show this container or label.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: C1OCOCO1
2. InChI: InChI=1S/C3H6O3/c1-4-2-6-3-5-1/h1-3H2
3. InChIKey: BGJSXRVXTHVRSN-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View