-
Name
3-Bromofluorobenzene
- EINECS 214-023-0
- CAS No. 1073-06-9
- Article Data23
- CAS DataBase
- Density 1.593 g/cm3
- Solubility insoluble in water
- Melting Point -8 °C
- Formula C6H4BrF
- Boiling Point 150 °C at 760 mmHg
- Molecular Weight 175
- Flash Point 38.9 °C
- Transport Information UN 1993 3/PG 3
- Appearance clear colourless tolight yellow liquid
- Safety 26-36-37/39-16
- Risk Codes 10-22-36/38-36/37/38-20/21/22
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1-Bromo-3-fluorobenzene;1-Fluoro-3-bromobenzene;3-Bromo-1-fluorobenzene;3-Fluoro-1-bromobenzene;3-Fluorobromobenzene;3-Fluorophenyl bromide;NSC10267;m-Bromofluorobenzene;m-Fluorobromobenzene;
- PSA 0.00000
- LogP 2.58820
Synthetic route
-
-
1430342-19-0
(3-fluorophenyl)(mesityl)iodonium trifluoromethanesulfonate

-
-
1073-06-9
3-fluorobromobenzene

| Conditions | Yield |
|---|---|
| With copper(I) bromide In acetonitrile at 80℃; for 2h; | 91% |

-
-
1073-06-9
3-fluorobromobenzene

| Conditions | Yield |
|---|---|
| With copper(I) oxide; sulfuric acid; sodium nitrite In water; isopropyl alcohol at -10 - 0℃; for 5h; Temperature; Reagent/catalyst; Solvent; | 82.7% |
-
-
400-31-7
1,1,1-trifluoro-4-methyl-pent-3-en-2-one

-
A
-
124445-98-3
2,2′-dibromo-4,4′-difluorobiphenyl

-
B
-
1073-06-9
3-fluorobromobenzene

-
C
-
1198785-52-2
4-(2-bromo-4-fluorophenyl)-1,1,1-trifluoro-4-methylpentan-2-one

-
D
-
1429053-26-8
2-(2-bromo-4-fluorophenyl)-1,1,1-trifluoro-4-methylpent-3-en-2-ol

-
E
-
496-69-5
2-bromo-4-fluoro-phenol

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromo-4-fluoro-1-iodobenzene With isopropylmagnesium chloride In tetrahydrofuran at -30 - -25℃; for 0.5h; Inert atmosphere; Large scale; Stage #2: 1,1,1-trifluoro-4-methyl-pent-3-en-2-one With copper(l) iodide In tetrahydrofuran at -30 - -25℃; for 4h; Inert atmosphere; Large scale; | A 0.5% B 16% C 75% D 5% E 0.5% |

-
-
108-86-1
bromobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1073-06-9
3-fluorobromobenzene

-
C
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| With fluorine In trichlorofluoromethane at -78℃; for 1h; | A 23 % Chromat. B 17 % Chromat. C 60 % Chromat. |
| With fluorine In trichlorofluoromethane at -78℃; for 1h; Rate constant; Product distribution; competitive reaction with benzene; | A 23 % Chromat. B 17 % Chromat. C 60 % Chromat. |
| With xenon difluoride; boron trifluoride diethyl etherate In acetonitrile at -35 - 20℃; for 2.5h; Inert atmosphere; Overall yield = 56 %; |


| Conditions | Yield |
|---|---|
| With bromobenzene; Cu-HZSM-5 zeolite at 399.9℃; Mechanism; various catalysts; | 14.3 % Chromat. |
-
-
75-15-0
carbon disulfide

-
-
462-06-6
fluorobenzene

-
-
7726-95-6
bromine

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1073-06-9
3-fluorobromobenzene

-
C
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| at 55℃; Product distribution; |

-
-
56-23-5
tetrachloromethane

-
-
462-06-6
fluorobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1073-06-9
3-fluorobromobenzene

-
C
-
352-34-1
4-fluoro-1-iodobenzene

-
D
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| Product distribution; |

-
-
462-06-6
fluorobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1073-06-9
3-fluorobromobenzene

-
C
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| With bromine at 25℃; for 16h; Irradiation; Yield given; |


-
-
1073-06-9
3-fluorobromobenzene

| Conditions | Yield |
|---|---|
| With bromine at 400 - 700℃; |
-
-
108-86-1
bromobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
1000995-64-1
2-bromo-1-oxypentafluorosulfanylbenzene

-
C
-
344455-90-9
4-bromo-1-oxypentafluorosulfanylbenzene

-
R
-
1073-06-9
3-fluorobromobenzene

-
S
-
108-36-1
1,3-dibromobenzene

-
T
-
106-37-6
1.4-dibromobenzene

-
U
-
460-00-4
1-Bromo-4-fluorobenzene

-
V
-
583-53-9
1,2-dibromobenzene

| Conditions | Yield |
|---|---|
| With bis(pentafluorosulfur) peroxide at 100℃; for 17h; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 3-chloro-benzenecarboperoxoic acid 2: copper(I) bromide / acetonitrile / 2 h / 80 °C View Scheme |
-
-
108-86-1
bromobenzene

-
A
-
1072-85-1
o-fluorobromobenzene

-
B
-
399-94-0
2-bromo-1,4-difluorobenzene

-
C
-
1073-06-9
3-fluorobromobenzene

-
D
-
348-61-8
1-bromo-3,4-difluorobenzene

-
E
-
460-00-4
1-Bromo-4-fluorobenzene

| Conditions | Yield |
|---|---|
| With xenon difluoride; boron trifluoride diethyl etherate at 0 - 25℃; Cooling with ice; | A 0.06 mmol B 0.01 mmol C 0.03 mmol D 0.004 mmol E 0.16 mmol |


-
-
100-52-7
benzaldehyde

-
A
-
1073-06-9
3-fluorobromobenzene

-
C
-
65-85-0
benzoic acid

-
D
-
100-51-6
benzyl alcohol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran at 40℃; for 1h; |


-
-
1073-06-9
3-fluorobromobenzene

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In tetrahydrofuran at 40℃; for 1h; |

| Conditions | Yield |
|---|---|
| Stage #1: 2-Fluoroaniline With sulfuric acid; dihydrogen peroxide; bromine at 10 - 15℃; for 0.5h; Stage #2: With nitrosylsulfuric acid at 5℃; for 2h; Overall yield = 80.8 %; |
-
-
1073-06-9
3-fluorobromobenzene

-
-
109-94-4
formic acid ethyl ester

-
-
98586-21-1
bis(3-fluorophenyl)methanol

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluorobromobenzene With magnesium In tetrahydrofuran Stage #2: formic acid ethyl ester In tetrahydrofuran at 0 - 20℃; | 100% |
| With magnesium In diethyl ether for 18h; Heating; | 95% |
| Stage #1: 3-fluorobromobenzene With n-butyllithium In tetrahydrofuran at -78℃; for 1h; Stage #2: formic acid ethyl ester In tetrahydrofuran at -78 - 0℃; for 1h; Further stages.; |
-
-
1073-06-9
3-fluorobromobenzene

-
-
93-61-8
N-methyl-N-phenylformamide

-
-
360575-28-6
2-bromo-6-fluorobenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluorobromobenzene With hydrogenchloride; n-butyllithium; diisopropylamine In tetrahydrofuran; hexanes at -78 - -70℃; for 2.5h; Stage #2: N-methyl-N-phenylformamide In tetrahydrofuran; hexanes at -78 - -70℃; for 2h; | 100% |
-
-
884510-86-5
(R)-tert-butyl 3-(methoxy(methyl)carbamoyl)piperidine-1-carboxylate

-
-
1073-06-9
3-fluorobromobenzene

-
-
942142-91-8
(R)-tert-butyl 3-(3-fluorobenzoyl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluorobromobenzene With magnesium In tetrahydrofuran at 50 - 60℃; for 1h; Stage #2: (R)-tert-butyl 3-(methoxy(methyl)carbamoyl)piperidine-1-carboxylate In tetrahydrofuran at -78 - 20℃; for 1.5h; Stage #3: With water; ammonium chloride In tetrahydrofuran | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluorobromobenzene; phenylboronic acid With potassium phosphate In isopropyl alcohol at 20℃; for 4h; Suzuki Coupling; Stage #2: [Pd2(μ-Cl)2{{(C2F5)2O}2H}2] In isopropyl alcohol at 20℃; for 20h; Suzuki Coupling; | 100% |
| Stage #1: 3-fluorobromobenzene; phenylboronic acid With potassium phosphate In isopropyl alcohol at 20℃; for 2h; Suzuki coupling; Inert atmosphere; Stage #2: With Juliphos In isopropyl alcohol at 20℃; for 20h; Suzuki coupling; Inert atmosphere; | 100% |
| With potassium phosphate tribasic trihydrate; 1-(tert-butyl)-2-(diphenylphosphaneyl)-1H-imidazole; palladium dichloride In ethanol at 60℃; for 1h; Suzuki Coupling; Inert atmosphere; Schlenk technique; | 97% |
-
-
1073-06-9
3-fluorobromobenzene

-
-
388061-72-1
3-[2-(trimethylsilylethynyl)]phenol

| Conditions | Yield |
|---|---|
| Stage #1: 3-[2-(trimethylsilylethynyl)]phenol With tetrabutyl ammonium fluoride In tetrahydrofuran at 20℃; for 0.5h; Stage #2: 3-fluorobromobenzene With bis-triphenylphosphine-palladium(II) chloride In tetrahydrofuran at 80℃; for 24h; | 100% |
-
-
1073-06-9
3-fluorobromobenzene

-
-
109-94-4
formic acid ethyl ester

-
-
98586-21-1
bis(3-fluorophenyl)methanol

| Conditions | Yield |
|---|---|
| With magnesium In tetrahydrofuran; water | 99% |


| Conditions | Yield |
|---|---|
| With N-[2-(pyrazol-1-yl)phenyl]-N'-benzylimidazol-2-ylidene palladium chloride; caesium carbonate In water; isopropyl alcohol Reagent/catalyst; Suzuki-Miyaura Coupling; | 99% |

| Conditions | Yield |
|---|---|
| With N-[2-(3,5-dimethylpyrazol-1-yl)phenyl]-N'-benzylimidazol-2-ylidene palladium chloride; potassium carbonate In water; N,N-dimethyl-formamide Reagent/catalyst; Heck Reaction; | 99% |
| With potassium phosphate; C34H46Br4N8Pd2 In N,N-dimethyl-formamide at 110℃; for 4h; Heck Reaction; | 82% |
| With C74H61Cl2N3Pd; triethylamine In 1,4-dioxane for 10h; Heck Reaction; | 77% |
-
-
1073-06-9
3-fluorobromobenzene

-
-
73183-34-3
bis(pinacol)diborane

| Conditions | Yield |
|---|---|
| With 10H-phenothiazine; caesium carbonate In acetonitrile for 48h; Irradiation; Sealed tube; Inert atmosphere; | 99% |
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate In dimethyl sulfoxide at 80℃; for 48h; Inert atmosphere; | 31% |
| With {meso-1,3-bis(1-methylethyl)-4,5-(9,10-dihydroanthraceno)imidazolin-2-ylidene}copper(I) chloride; potassium tert-butylate In tert-butyl methyl ether at 25℃; for 4h; Inert atmosphere; | 63 %Spectr. |
| With (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride; potassium acetate In 1,4-dioxane at 110℃; for 0.166667h; Miyaura Borylation Reaction; Microwave irradiation; |
-
-
1073-06-9
3-fluorobromobenzene

-
-
143360-89-8
o-(phenylethynyl)trifluoroacetanilide

| Conditions | Yield |
|---|---|
| With caesium carbonate; tetrakis(triphenylphosphine) palladium(0) In acetonitrile at 100℃; for 0.5h; | 98% |
| With caesium carbonate; tetrakis(triphenylphosphine) palladium(0) In acetonitrile at 100℃; for 3h; | 98% |
-
-
1436-43-7
quinoline-2-carbonitrile

-
-
1073-06-9
3-fluorobromobenzene

-
-
1177408-13-7
(3-fluorophenyl)(quinolin-2-yl)methanone

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluorobromobenzene With magnesium In tetrahydrofuran Inert atmosphere; Stage #2: quinoline-2-carbonitrile In tetrahydrofuran; toluene at 0 - 20℃; Inert atmosphere; Stage #3: With hydrogenchloride; water In diethyl ether for 0.333333h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; palladium diacetate; di-tert-butyl (2'-methyl-[1,1'-biphenyl]-2-yl)phosphine In 1,4-dioxane; tert-Amyl alcohol Inert atmosphere; Reflux; | 98% |
| With potassium phosphate; palladium diacetate; di-tert-butyl (2'-methyl-[1,1'-biphenyl]-2-yl)phosphine In 1,4-dioxane; tert-Amyl alcohol Inert atmosphere; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; palladium diacetate; potassium carbonate In ethanol; water at 40 - 72℃; for 4h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); caesium carbonate In tetrahydrofuran; water at 80℃; for 10h; Inert atmosphere; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluorobromobenzene With 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl; bis(dibenzylideneacetone)-palladium(0) In 1,4-dioxane Inert atmosphere; Stage #2: 3,6-dioxa-1,8-diaminooctane With sodium t-butanolate In 1,4-dioxane for 8h; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With Na2{PdCl2(Ph2P(m-C6H4SO3))2}; sodium 3-(diphenylphosphanyl)benzenesulfonate; trimethyloctadecylammonium chloride; 1,8-diazabicyclo[5.4.0]undec-7-ene In water for 20h; Heating; | 97% |
| With bis-triphenylphosphine-palladium(II) chloride; 1,8-diazabicyclo[5.4.0]undec-7-ene; 1,4-di(diphenylphosphino)-butane In dimethyl sulfoxide at 80℃; for 16h; Inert atmosphere; |
-
-
1073-06-9
3-fluorobromobenzene

-
-
1523523-71-8
1-(S-phenylsulfonimidoyl)piperidine

| Conditions | Yield |
|---|---|
| With palladium diacetate; caesium carbonate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In toluene at 20 - 100℃; Inert atmosphere; | 97% |
-
-
1073-06-9
3-fluorobromobenzene


| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); dicyclohexyl-(2′,4′,6′-triisopropyl-3,6-dimethoxy-[1,1′-biphenyl]-2-yl)phosphine; sodium t-butanolate In 1,4-dioxane at 110℃; for 2h; Inert atmosphere; | 96% |
-
-
540-88-5
acetic acid tert-butyl ester

-
-
1073-06-9
3-fluorobromobenzene

-
-
476429-07-9
tert-butyl α-(3-fluorophenyl)acetate

| Conditions | Yield |
|---|---|
| With tri-tert-butyl phosphine; lithium dicyclohexylamide; bis(dibenzylideneacetone)-palladium(0) In toluene at 20℃; for 15h; | 95% |

| Conditions | Yield |
|---|---|
| at 20℃; for 3h; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-methylquinoline With zinc chloride-2,2,6,6-tetramethylpiperidin-1-ide lithium chloride complex In tetrahydrofuran at 25℃; for 1h; Inert atmosphere; Stage #2: 3-fluorobromobenzene With palladium diacetate; 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In tetrahydrofuran at 50℃; for 1h; Inert atmosphere; | 95% |
-
-
1073-06-9
3-fluorobromobenzene

-
-
931-50-0
cyclohexylmagnesium bromide

-
-
1717-83-5
1-cyclohexyl-3-fluorobenzene

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluorobromobenzene With dichloro [1,1'-bis(diphenylphosphino)propane]palladium(II) In tetrahydrofuran for 0.0833333h; Suzuki-Miyaura Coupling; Inert atmosphere; Schlenk technique; Stage #2: cyclohexylmagnesium bromide With lithium bromide In tetrahydrofuran at 20℃; for 24h; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 95% |

| Conditions | Yield |
|---|---|
| With chloro[1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene]copper(I); 4,4,5,5-tetramethyl-2-(4,4,5,5-tetramethyl-1,3-dioxolan-2-yl)-1,3,2-dioxaborolane; [PdCl2(N,N’-bis-[2,6-(diisopropyl)phenyl]imidazolidin-2-ylidene){P(N,N’-bis-[2,6-(diisopropyl)phenyl]imidazol-2-ylidene)3}]; potassium 2-methylbutan-2-olate In ethanol at 80℃; for 20h; Glovebox; regioselective reaction; | 95% |
-
-
1073-06-9
3-fluorobromobenzene

-
-
87199-17-5
4-formylphenylboronic acid,

-
-
400750-63-2
3'-fluoro[1,1'-biphenyl]-4-carbaldehyde

| Conditions | Yield |
|---|---|
| With C5H6N2*Pd(2+)*2Cl(1-); potassium carbonate In ethanol; water for 0.166667h; Suzuki-Miyaura Coupling; Reflux; Schlenk technique; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluorobromobenzene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 1h; Inert atmosphere; Schlenk technique; Stage #2: 2-bromo-4-methylbenzaldehyde In tetrahydrofuran; hexane at -78 - 20℃; for 2h; Inert atmosphere; Schlenk technique; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-fluorobromobenzene With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.0833333h; Inert atmosphere; Stage #2: phenyl[(2-phenylsulfanyl)phenyl]methanone In tetrahydrofuran; hexane at -78℃; Inert atmosphere; | 95% |
-
-
1073-06-9
3-fluorobromobenzene

-
-
25015-63-8
4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane

-
-
1357266-25-1
2-(3-bromo-5-fluorophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| Stage #1: 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer In tetrahydrofuran at 20℃; Glovebox; Inert atmosphere; Stage #2: With 2,2'-methylenebis( N,N)-dimethylpyridin-4-amine In tetrahydrofuran at 20℃; for 0.25h; Glovebox; Inert atmosphere; Stage #3: 3-fluorobromobenzene In tetrahydrofuran at 20℃; for 5h; Glovebox; Inert atmosphere; regioselective reaction; | 95% |

| Conditions | Yield |
|---|---|
| With tri-tert-butyl phosphine; lithium dicyclohexylamide; bis(dibenzylideneacetone)-palladium(0) In toluene at 20℃; for 24h; | 94% |
-
-
1073-06-9
3-fluorobromobenzene

-
-
536-74-3
phenylacetylene

-
-
29778-28-7
1-fluoro-3-(2-phenylethynyl)benzene

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; sodium acetate In dimethyl sulfoxide at 110℃; for 35h; Sonogashira Cross-Coupling; Inert atmosphere; | 94% |
| With potassium phosphate; C30H37Br2N3Pd(2-) In dimethyl sulfoxide at 100℃; for 1h; Sonogashira Cross-Coupling; | 84% |
| With bis-triphenylphosphine-palladium(II) chloride; copper(l) iodide; diisopropylamine; tri tert-butylphosphoniumtetrafluoroborate In 1,4-dioxane at 20℃; Schlenk technique; Inert atmosphere; | 83% |
| With C34H40Br2N3Pd; potassium carbonate In dimethyl sulfoxide at 100℃; for 1h; Sonogashira coupling; | 59% |
| With Sonogashira catalyst CMX-1 In HN(iPr)2 at 80℃; Sonogashira coupling; |
-
-
58-08-2
3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione

-
-
1073-06-9
3-fluorobromobenzene

-
-
1161823-22-8
1,3,7-trimethyl-8-(m-fluorophenyl)-xanthine

| Conditions | Yield |
|---|---|
| With potassium phosphate; copper(l) iodide; 1,10-Phenanthroline In 5,5-dimethyl-1,3-cyclohexadiene; N,N-dimethyl-formamide at 20 - 140℃; for 36.1667h; Inert atmosphere; regioselective reaction; | 94% |
3-Bromofluorobenzene Chemical Properties
IUPAC Name: 1-bromo-3-fluorobenzene
Empirical Formula: C6H4BrF
Molecular Weight: 174.9984g/mol
EINECS: 214-023-0
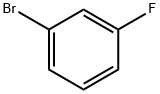
Structure of Benzene,1-bromo-3-fluoro- (CAS NO.1073-06-9):
Index of Refraction: 1.53
Molar Refractivity: 33.93 cm3
Molar Volume: 109.8 cm3
Polarizability: 13.45×10-24cm3
Surface Tension: 33.8 dyne/cm
Density: 1.593 g/cm3
Flash Point: 38.9 °C
Enthalpy of Vaporization: 37.09 kJ/mol
Melting Point: -8°C
Boiling Point: 150 °C at 760 mmHg
Vapour Pressure: 4.99 mmHg at 25°C
storage temp: Flammables area
Water Solubility: insoluble
Product Categories: Aromatic Hydrocarbons (substituted) & Derivatives;Benzene series;Fluorobenzene;Bromine Compounds;Fluorine Compounds;Fluorobenzene Series;Aryl;C6;Halogenated Hydrocarbons
Canonical SMILES: C1=CC(=CC(=C1)Br)F
InChI: InChI=1S/C6H4BrF/c7-5-2-1-3-6(8)4-5/h1-4H
InChIKey: QDFKKJYEIFBEFC-UHFFFAOYSA-N
3-Bromofluorobenzene Uses
Intermediates of Liquid Crystals.
3-Bromofluorobenzene Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MOD | 28ZPAK Sbornik Vysledku Toxixologickeho Vysetreni Latek A Pripravku Marhold, J.V.,Institut Pro Vychovu Vedoucicn Pracovniku Chemickeho Prumyclu Praha,Czechoslovakia.: 1972,32. | ||
| 2. | eye-rbt 20 mg/24H MOD | 28ZPAK Sbornik Vysledku Toxixologickeho Vysetreni Latek A Pripravku Marhold, J.V.,Institut Pro Vychovu Vedoucicn Pracovniku Chemickeho Prumyclu Praha,Czechoslovakia.: 1972,32. |
3-Bromofluorobenzene Consensus Reports
Reported in EPA TSCA Inventory.
3-Bromofluorobenzene Safety Profile
A skin and eye irritant. When heated to decomposition it emits very toxic fumes of F− and Br−.
The Hazard Codes of Benzene,1-bromo-3-fluoro- (1073-06-9):  Xn,
Xn, Xi,
Xi,  F
F
Hazard Note: Flammable/Irritant
HazardClass: 3
The Risk Statements information of Benzene,1-bromo-3-fluoro- (1073-06-9):
10: Flammable
22: Harmful if swallowed
20/21/22: Harmful by inhalation, in contact with skin and if swallowed
36/37/38: Irritating to eyes, respiratory system and skin
36/38: Irritating to eyes and skin
The Safety Statements information of Benzene,1-bromo-3-fluoro- (1073-06-9):
16: Keep away from sources of ignition - No smoking
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37/39: Wear suitable gloves and eye/face protection
RIDADR: UN 1993 3/PG 3
WGK Germany: 2
RTECS: DA1225000
PackingGroup: III
HS Code: 29036990
3-Bromofluorobenzene Specification
Benzene,1-bromo-3-fluoro- , its cas register number is 1073-06-9. It also can be called 1-Bromo-3-fluorobenzene ; 1-Fluoro-3-bromobenzene ; 3-Bromfluorbenzen ; 3-Bromofluorobenzene ; m-Bromofluorobenzene ; m-Fluorobromobenzene ;
m-Fluorophenyl bromide . Benzene,1-bromo-3-fluoro- (CAS NO.1073-06-9) is a clear colourless tolight yellow liquid.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View