Synthetic route
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) byproducts: MgCl2; heating at 900-1100°C;; | 98% |
Conditions
| Conditions | Yield |
|---|
| With zinc In melt Electrolysis; melting electrolysis, Zn-electrode, addition of alkalichloride;; evaporation of Zn in vacuum at 700-800°C;; | 98% |
| With zinc In melt Electrolysis; melting electrolysis, Zn-electrode, addition of alkalichloride;; evaporation of Zn in vacuum at 700-800°C;; | 98% |
| With magnesium; sodium chloride In melt heating in an iron crucible, decanting of salt melt, addition of water and HCl to Zr residue after cooling down;; impured;; | 88% |
-
Zn(b),Zr(15) (X%)
-
Zn(b),Zr(15) (X%)
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) heating in vacuum at 800°C for several hours;; 98 % Zr with 0.05 % Zn;; | 98% |
-
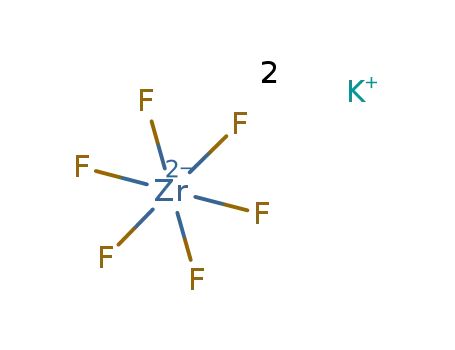
-
potassium hexafluorozirconate
Conditions
| Conditions | Yield |
|---|
| With sodium In neat (no solvent) heating at higher temperature 30 - 40 min in a closed evacuated copper vessel; mixture filled under ether;; | 93.4% |
| With potassium chloride; sodium; sodium chloride In melt byproducts: H2; slow addition of K2ZrF6 and NaCl, KCl to Na melt, heating in iron vessel at 800°C under H2, stirring, further heating for 2.5 hours;; crushing, washing with water; solvation of iron impurities by aq. HCl, drying at 60°C;; | 90% |
| With sodium In neat (no solvent) heating with blow pipe 30 - 40 min in a closed evacuated copper-vessel; mixture filled under ether;; | 80% |
Conditions
| Conditions | Yield |
|---|
| In melt heating at 800°C under inert gas in a closed vessel, Kroll-process; apparatus described;; separation of Mg and MgCl2 by vacuum destillation;; | A 93%
B n/a |
| In melt heating at 800°C under inert gas in a closed vessel, Kroll-process; apparatus described;; separation of Mg and MgCl2 by vacuum destillation;; | A 93%
B n/a |
-
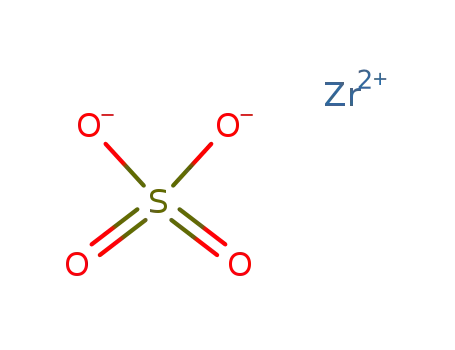
-
zirconium sulphate
Conditions
| Conditions | Yield |
|---|
| With tannine or saponine In water Electrolysis; electrolysis on Cr cathode, keeping react. product in air Zr separated through diffusion; | |
| With tannine or saponine In water Electrolysis; electrolysis on Cr cathode, keeping react. product in air Zr separated through diffusion; | |
-
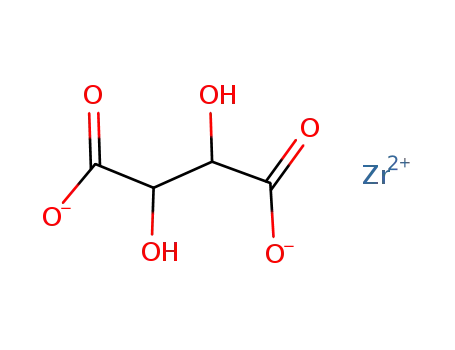
-
Zr-tartrate
Conditions
| Conditions | Yield |
|---|
| With tannine or saponine In water Electrolysis; electrolysis on Cr cathode, keeping react. product in air Zr separated through diffusion; | |
| With tannine or saponine In water Electrolysis; electrolysis on Cr cathode, keeping react. product in air Zr separated through diffusion; | |
-

-
potassium chloride
Conditions
| Conditions | Yield |
|---|
| In melt Electrolysis; smelting of 60.2% AlCl3, 33.3% KCl, 0.4% ZrO2 and 6,0% NaF, graphite electrodes, tension of decomposition 1.0 V at 365°C;; | |
| In melt Electrolysis; smelting of 60.2% AlCl3, 33.3% KCl, 0.4% ZrO2 and 6,0% NaF, graphite electrodes, tension of decomposition 1.0 V at 365°C;; | |
-
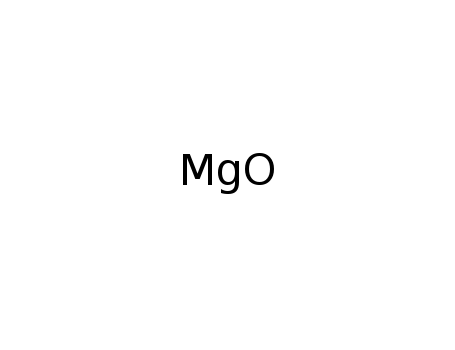
-
magnesium oxide
Conditions
| Conditions | Yield |
|---|
| With magnesium In neat (no solvent) heating mixture of ZrO2 and Mg-foil in an iron tube under salt layer to red heat;; | |
| With magnesium In melt calcinating in a closed vessel;; fine black powder; solvation of MgO in aq. HCl;; | |
-

-
zirconium monochloride
Conditions
| Conditions | Yield |
|---|
| With Li In not given 850°C, 1 days; | |
-
-
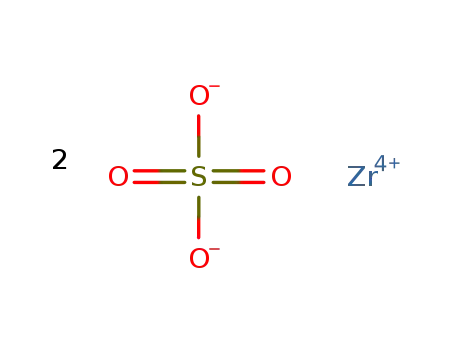
zirconium(IV) sulfate
Conditions
| Conditions | Yield |
|---|
| In methanol formation of a white precipitation;; | 0% |
Conditions
| Conditions | Yield |
|---|
| in H2 atmosphere; incomplete reaction; also formation of Mg zirconite; optimal yields at initiation with thermite mixtures; no formation of ZrO;; | |
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) byproducts: CaO; heating at 1050°C with Ca vapor;; | |
-
B
-
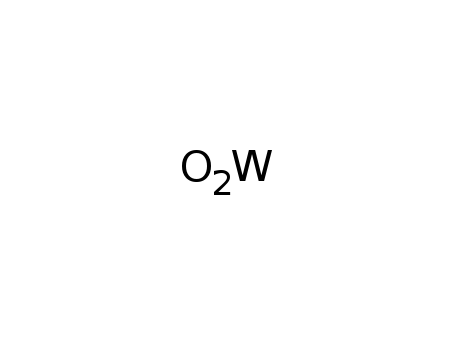
tungsten(IV) oxide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) heating at 2400-2500°C under N2; at lower temperatures back reaction to solid W and gaseous ZrO2;; | |
-

-
zirconium(IV) sulfate
Conditions
| Conditions | Yield |
|---|
| In methanol Zn is covered by a dark layer of Zr;; | |
-
ZrH(0.44+x) x:0-0.20;
-
ZrH(0.44+x) x:0-0.20;
Conditions
| Conditions | Yield |
|---|
| byproducts: H2; outgassing (800°C, vac. 1E-4 Pa); | |
Conditions
| Conditions | Yield |
|---|
| With magnesium; aluminium trichloride In neat (no solvent) reduction at 300-350°C;; ZrCl3 containing Mg crystals and small amount of Zr;; | |
| With Mg; aluminium trichloride In neat (no solvent) reduction at 300-350°C;; ZrCl3 containing Mg crystals and small amount of Zr;; | |
-
B

-
zirconium monochloride
Conditions
| Conditions | Yield |
|---|
| With AgCl In melt Electrochem. Process; under Ar; deposition of Zr and small amt. of ZrCl on Ta (working electrode), mixt. of KCl-LiCl eutectic, AgCl (1 wt.-%), and ZrCl4 heated to 450-550°C, 40-90 mA, -1.42 to -1.35 V vs Ag/AgCl, Cd pool as counterelectrode; XRD; | |
-
B

-
zirconium monocarbide
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) decomposition on a coal resistance wire; product mixture depending on temp. of wire;; | |
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) formation of Zr on W-wires at 2600°C, but the wire melts;; | |
| With H2 In neat (no solvent) formation of Zr on W-wires at 900-1400°C;; | |
| In neat (no solvent) formation of Zr on W-wires at 2600°C, but the wire melts;; | |
| With H2 In neat (no solvent) formation of Zr on W-wires at 900-1400°C;; | |
Conditions
| Conditions | Yield |
|---|
| With Na or Mg or Fe In not given calcination;; | |
| With Na or Mg or Fe In not given calcination;; | |
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Electric Arc; continuous process;; | |
| With hydrogen In neat (no solvent) byproducts: HI; formation of Zr on wires at 1100°C;; Addn. of H2 has no noticeable effect, since the product HI dissociates almost completely;; | |
| In neat (no solvent) heating in vacuum to about 600 °C, wire temp. <= 1900 °C;; formation of ZnN and Zr-hydrides is avoided by carrying out the process in vac.; at wire temps. > 1900 ° C the wire melts because of formation of a Zr-W eutectic;; | |
-
-
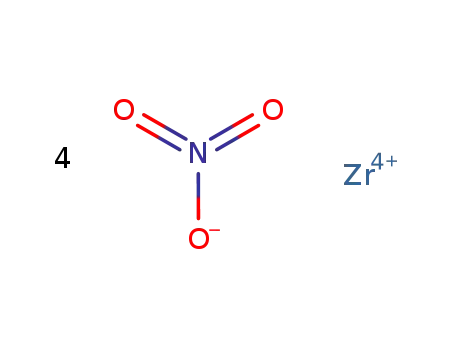
zirconium(IV) nitrate
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Electric Arc; heating in a W-vessel under inert gas;; | |
| In neat (no solvent) Electric Arc; heating in a W-vessel under inert gas;; | |
-

-
zirconium monocarbide
Conditions
| Conditions | Yield |
|---|
| 0% |
| In melt Zr, ZrO2 and ZrC are molten in high vacuum, whirling motion of charge by induction;; | |
| In neat (no solvent) heating in vacuum at 1960°C;; | 0% |
| In neat (no solvent) heating in vacuum at 1960°C;; | 0% |
| In melt Zr, ZrO2 and ZrC are molten in high vacuum, whirling motion of charge by induction;; | |
-

-
zirconium carbide
Conditions
| Conditions | Yield |
|---|
| With Zr In melt byproducts: CO; addition of ZrO2 to a molten mixture of ZrC and Zr;; the formed CO is continuous removed in vacuum;; | |
-
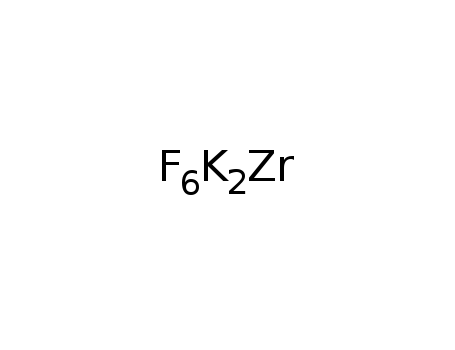
-
K2ZrF6
-
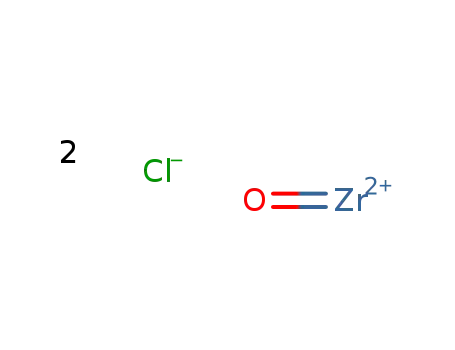
-
zirconyl chloride
Conditions
| Conditions | Yield |
|---|
| In water Electrolysis; precipitation;; | |
-

-
beryllium
-
![Na4[(Zr6Be)Cl16]](//file1.lookchem.com/cas/reactions/2021/05/25/16530647.png_ms)
-
Na4[(Zr6Be)Cl16]
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) stoichiometric mixture, sealed Ta container, 800-850°C; | 100% |
| In neat (no solvent) heating of stoich. amts. of reagents in a sealed Ta tube at 700-860°C for 2-3 weeks; identified by single crystal X-ray diffraction; | >90 |
| In neat (no solvent) mixt. of Zr, ZrCl4, NaCl and Be was sealed under Ar in Nb or Ta ampule; heated; | |
-

-
zirconium nitride chloride
-
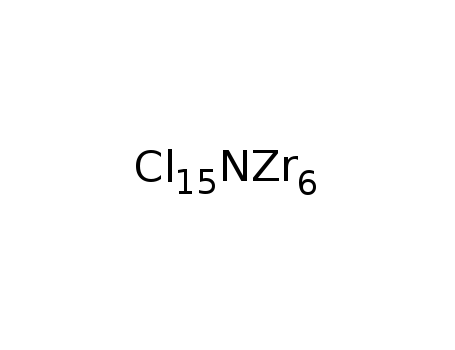
-
Zr6Cl15N
Conditions
| Conditions | Yield |
|---|
| prepn. in a sealed tantal tube at 700 °C for 2 weeks; | 100% |
| In neat (no solvent) heating of stoich. amts. of reagents in a sealed Ta tube at 700-860°C for 2-3 weeks; identified by single crystal X-ray diffraction; | >90 |
-

-
ZrNiSb
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) Electric Arc; heating (650°C, 4 d; dynamic Ar atm., arc melting), annealing (5 h, 1050-1200°C); | 100% |
| In neat (no solvent) vac. (5E-3 mbar); stoichiometric ratio, heating (1 week, 1100°C); | |
| In melt Electric Arc; arc melted under Ar gettered with Ti; 5 wt.-% of Sb required to compensate evaporative losses during arc-melting; ingots sealed in evacuated fused-silica tubes and annealed at 870 K for 720 h; quenched in cold water; XRD; EDX; | |
-

-
hafnium
-

-
niobium
-

-
Hf0207Nb0.21Ti0191V0.187Zr0206
Conditions
| Conditions | Yield |
|---|
| Inert atmosphere; Electric arc; | 99.9% |
-
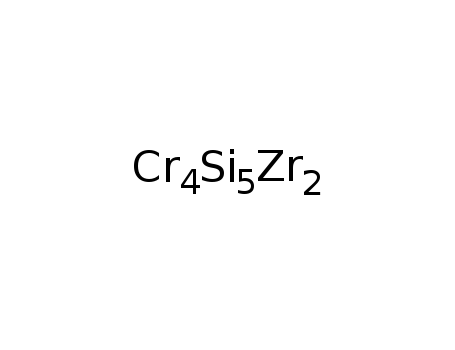
-
Zr2Cr4Si5
Conditions
| Conditions | Yield |
|---|
| In melt Zr, Cr, and Si were pressed into pellets and arc-melted under Ar; X-ray powder diffraction; | 99% |
-
-
thallium
-
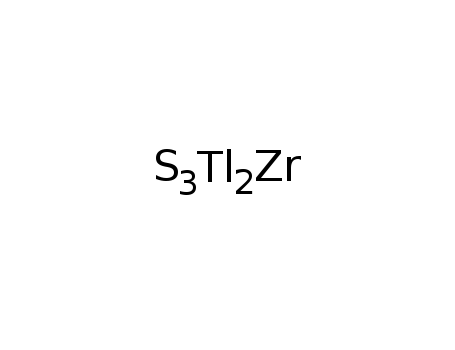
-
Tl2ZrS3
Conditions
| Conditions | Yield |
|---|
| In melt sealed under vac., heated to 1248 K in 72 h with intermittent halfs at 623 and 748 K for 24 h, kept at 1248 K for 10 h; cooled down to 848 K in 2 wk, furnace-cooled down to room temp.; | 99% |
-

-
thallium
-

-
Tl2ZrSe3
Conditions
| Conditions | Yield |
|---|
| In melt sealed under vac., heated to 1248 K in 72 h with intermittent halfs at 623 and 748 K for 24 h, kept at 1248 K for 10 h; cooled down to 848 K in 2 wk, furnace-cooled down to room temp.; obtained slightly impure; | 99% |
-

-
beryllium
-

-
rubidium chloride
-
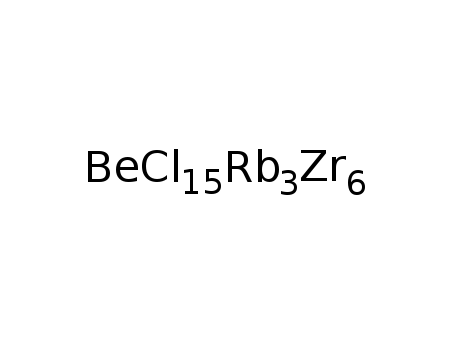
-
Rb3Zr6Cl15Be
Conditions
| Conditions | Yield |
|---|
| 800 ° C and quenched after 21 d; | 95% |
-
-
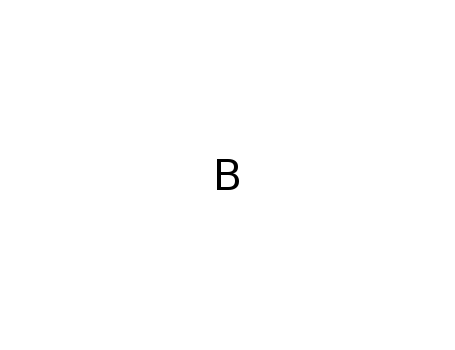
boron
-

-
potassium chloride
Conditions
| Conditions | Yield |
|---|
| 850 ° C for 29 d; finally air-quenched; | 95% |
| In neat (no solvent) heating of stoich. amts. of reagents in a sealed Ta tube at 700-860°C for 2-3 weeks; identified by single crystal X-ray diffraction; | >90 |
-

-
beryllium
-

-
potassium chloride
-
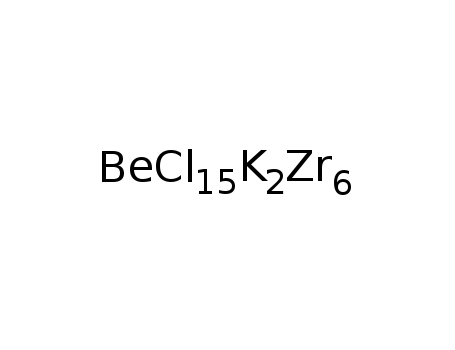
-
K2Zr6Cl15Be
Conditions
| Conditions | Yield |
|---|
| 800-850 ° C over a 2-3 week period; | 95% |
-

-
lithium chloride
-

-
iron(II) chloride
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) sublimed ZrCl4, FeCl3, LiCl, and Zr in stoich. proportions are heated at 800-850°C; crystal growth in temp. gradient react. 910-890°C, 25 d; | 95% |
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) sublimated ZrCl4, CoCl2, and Zr in stoich. proportions are heated at 850°C for 30 days; | 95% |
| With KCl; BaCl2 educts heated in KBaZr6Cl18Co stoich. proportions at 750°C for 15 days; detd. Guinier powder diffraction; | |
-
A
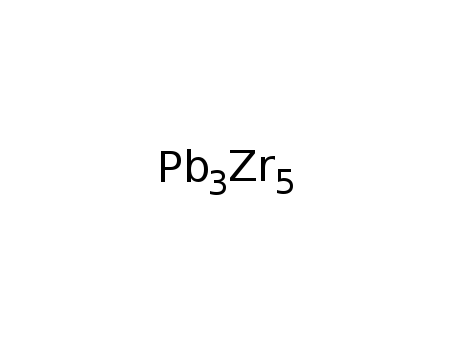
-
Zr5Pb3
-
B
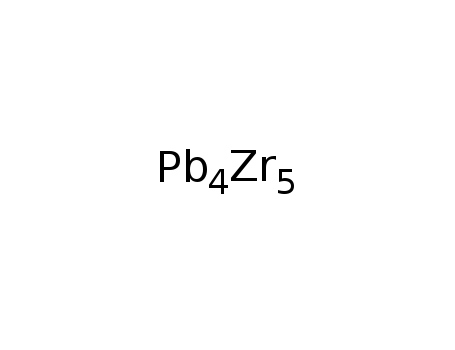
-
Zr5Pb4
Conditions
| Conditions | Yield |
|---|
| In melt heating at 700°C in sealed Ta tubes, 7d; | A 5%
B 95% |
| In melt heating at 800°C in sealed Ta tubes, 7d; | A 60%
B 40% |
-

-
boron
-
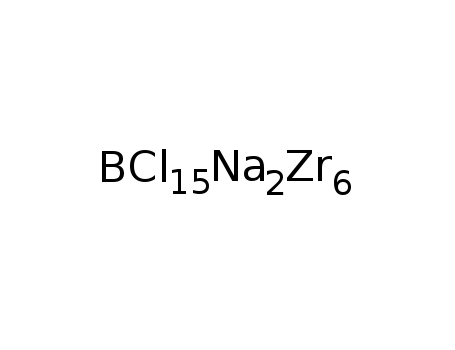
-
Na2Zr6Cl15B
Conditions
| Conditions | Yield |
|---|
| With NaCl reactn. at 850 °C; | 95% |
-

-
beryllium
-

-
potassium chloride
Conditions
| Conditions | Yield |
|---|
| byproducts: K2ZrCl6; 800 ° C and quenched after 21 d; | 95% |
-

-
lithium chloride
-

-
manganese(ll) chloride
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) sublimed ZrCl4, MnCl2, LiCl, and Zr in stoich. proportions are heated at 800-850°C; crystal growth in temp. gradient react. at 950-800°C for 24 d; | 95% |
-

-
manganese(ll) chloride
-

-
NaZr6Cl14Mn
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) react. in a sealed Ta tube at ca 750°C; Zhang, J. and Corbett, J. D., Inorg. Chem. 32 (1993), p. 1566; | 90% |
| In neat (no solvent) byproducts: ZrCl; react. in a sealed Ta tube at ca 800°C; Zhang, J. and Corbett, J. D., Inorg. Chem. 32 (1993), p. 1566; | |
-

-
boron
-

-
cesium chloride
-
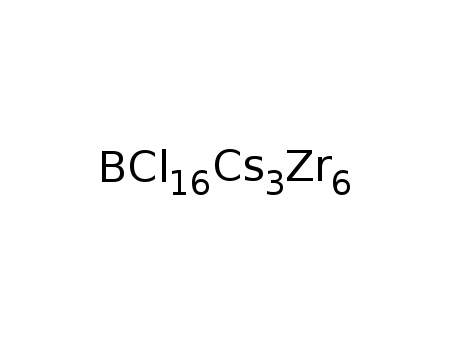
-
Cs3Zr6Cl16B
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) stoichiometric mixture, sealed Ta container, 850°C; | 90% |
-

-
beryllium
-

-
cesium chloride
-
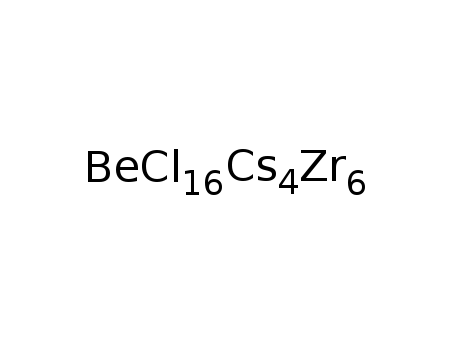
-
Cs4Zr6Cl16Be
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) byproducts: Cs2ZrCl16; stoichiometric mixture, sealed Ta container, 850°C, 2 weeks; | 90% |
-
-
nickel dichloride
Conditions
| Conditions | Yield |
|---|
| With KCl In neat (no solvent) byproducts: K2ZrCl6; educts heated in K2Zr6Cl18Ni stoich. proportions at 850°C for 25 d; crystal growth in temp. gradient react. 750-650°C for 7 d; | 90% |
| With CsCl In neat (no solvent) byproducts: Cs2ZrCl6; in Cs2Zr6Cl18Ni stoich. proportions heated at 850°C for 25 d; crystal growth in temp. gradient react. 750-650°C for 7 d; | 80% |
-
-
graphite
-

-
cesium chloride
-
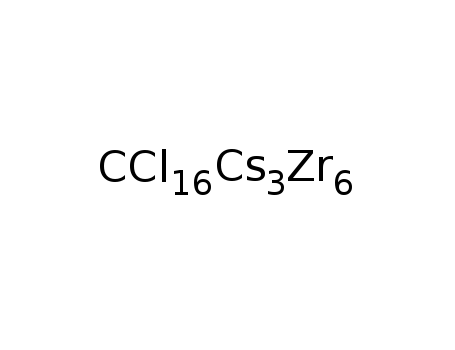
-
Cs3Zr6Cl16C
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) stoichiometric mixture, sealed Ta container, 850°C; | 90% |
-
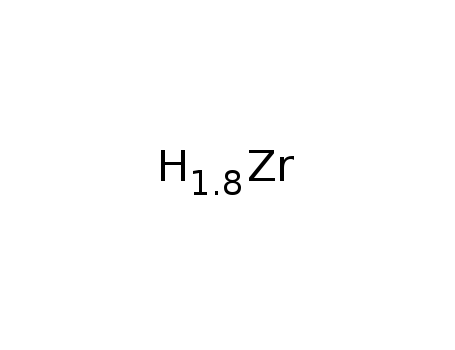
-
ZrH1.80
-
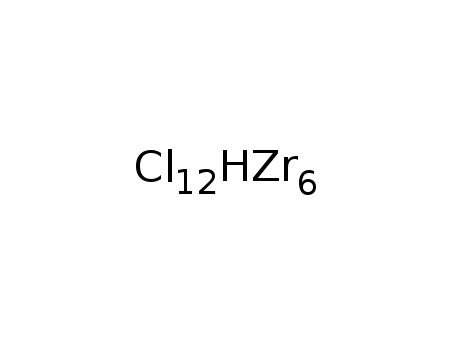
-
Zr6Cl12H
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) reactants under inert atmosphere or vacuum, Zr:Cl:H = 6:12:4 >600°C, 2-3 weeks, welded Ta containers in evacuated and sealed fused-silica jackets, 5-10 atm, by-product: ZrClOxHy (0; | 90% |
| In neat (no solvent) reactants under inert atmosphere or vacuum, Zr:Cl:H = 6:12:1.8 >600°C, 2-3 weeks, welded Ta containers in evacuated and sealed fused-silica jackets, 5-10 atm, by-product: ZrClOxHy (0; | 85% |
| In neat (no solvent) 700-750°C, 10-14 d; | 70-80 |
-

-
graphite
-
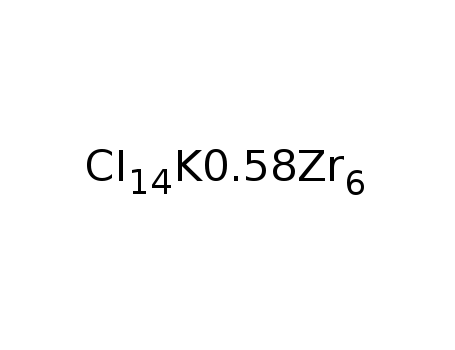
-
K0.58Zr6I14C
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent, solid phase) react. of stoich. quantities of Zr powder, ZrI4, graphite, and KI to form KZr6I14C in sealed Ta tube at 850°C; | 90% |
-
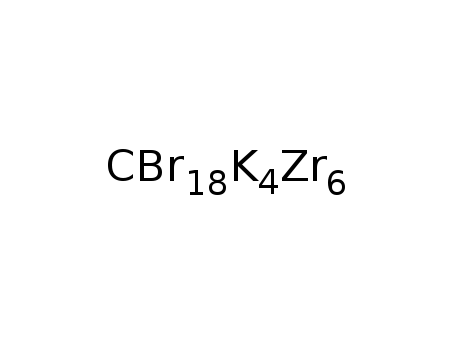
-
K4Zr6Br18C
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) heating (820°C, 4 weeks); | 90% |
Conditions
| Conditions | Yield |
|---|
| With (C4H9)4NBF4 In acetonitrile Electrolysis; reaction of Zr anode with 30% soln. of acetylacetone in CH3CN with added 0.1 M Bu4NBF4, Pt cathode; addn. of ether-acetone mixture;; pptn.; drying in vacuo; crystallization from CH3CN;; | 87% |
Zirconium Chemical Properties
Molecular Structure:
.png)
Molecular Formula: Zr
Molecular Weight: 91.224
IUPAC Name: Zirconium
Synonyms of Zirconium (CAS NO.7440-67-7): EINECS 231-176-9 ; HSDB 2528 ; Zircat ; Zirconium metal, dry, chemically produced, finer than 20 mesh particle size (UN2008) ; UN1358 ; UN1932 ; UN2008 ; UN2009 ; UN2858 ; Zirconium and compounds ; Zirconium compounds ; Zirconium powder ; Zirconium powder, dry ; Zirconium powder, dry [UN2008] [Spontaneously combustible] ; Zirconium powder, wetted with not <25% water (a visible excess of water must be present) (a) mechanically produced, particle size <53 microns, (b) chemically produced, particle size <840 microns ; Zirconium powder, wetted with not <25% water (a visible excess of water must be present) (a) mechanically produced, particle size <53 microns, (b) chemically produced, particle size <840 microns [UN1358] [Flammable solid] ; Zirconium scrap ; Zirconium scrap [UN1932] [Spontaneously combustible] Zirconium, dry, coiled wire, finished metal sheets, strip (thinner than 254 microns but not thinner than 18 microns) ; Zirconium, dry, coiled wire, finished metal sheets, strip (thinner than 254 microns but not thinner than 18 microns) [UN2858] [Flammable solid] ; Zirconium, dry, finished sheets, strip or coiled wire ; Zirconium, dry, finished sheets, strip or coiled wire [UN2009] [Spontaneously combustible] ; Zirconium, elemental
CAS NO: 7440-67-7
Classification Code: TWA 5 mg/m3 ; TWA 5 mg/m3; STEL 10 mg/m3; Not classifiable as a human carcinogen
Melting point: 1852 °C
Boiling Point: 4377 °C
Density: 1.01 g/mL at 25 °C
Form: wire
Merck: 13,10226
Zirconium History
The mineral was not known to contain a new element until 1789, when Klaproth analyzed a jargoon from the island of Sri Lanka in the Indian Ocean. He named the new element Zirkonerde (zirconia). Zirconium was first isolated in an impure form in 1824 by Berzelius by heating a mixture of potassium and potassium-zirconium fluoride in a small decomposition process conducted in an iron tube.
The crystal bar process (or Iodide process), discovered by Anton Eduard van Arkel and Jan Hendrik de Boer in 1925. It was superseded in 1945 by the much cheaper Kroll process developed by William Justin Kroll, in which zirconium tetrachloride is broken down by magnesium.
Zirconium Uses
Zirconium (CAS NO.7440-67-7) is used in weapons such as the BLU-97/B Combined Effects Bomb for incendiary effect. It is used as an alloying agent due to its high resistance to corrosion. It is also a component in some abrasives, such as grinding wheels and sandpaper.
Zirconium Consensus Reports
Reported in EPA TSCA Inventory.
Zirconium Safety Profile
Hazard Codes of Zirconium (CAS NO.7440-67-7):  F,
F, Xi
Xi
Risk Statements: 17-15-36/37/38
R17: Spontaneously flammable in air.
R15: Contact with water liberates extremely flammable gases.
R36/37/38: Irritating to eyes, respiratory system and skin.
Safety Statements: 26-43-7/8-36/37/39-35-27-16
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S43: In case of fire use ... (there follows the type of fire-fighting equipment to be used.)
S7: Keep container tightly closed.
S8: Keep container dry.
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
S27: Take off immediately all contaminated clothing.
S16: Keep away from sources of ignition.
RIDADR: UN 2858 4.1/PG 3
WGK Germany: 2
RTECS: ZH7070000
F: 10
HazardClass: 4.2
PackingGroup: III
A very dangerous fire hazard in the form of dust when exposed to heat or flame or by chemical reaction with oxidizers. May ignite spontaneously. A dangerous explosion hazard in the form of dust by chemical reaction with air, alkali hydroxides, alkali metal chromates, dichromates, molybdates, sulfates, tungstates, borax, CCl4, CuO, Pb, PbO, P, KClO3, KNO3, nitrylfluoride. Explosive range: 0.16 g/L in air. To fight fire, use special mixtures, dry chemical, salt, or dry sand. See also ZIRCONIUM COMPOUNDS.
Zirconium Standards and Recommendations
OSHA PEL: TWA 5 mg(Zr)/m3; STEL 10 mg(Zr)/m3; Not Classifiable as a Human Carcinogen
ACGIH TLV: TWA 5 mg(Zr)/m3; STEL 10 mg(Zr)/m3; Not Classifiable as a Human Carcinogen
DFG MAK: 1 mg(Zr)/m3
DOT Classification: 4.1; Label: Flammable Solid (UN 2858, UN 1358); DOT Class: 4.2; Label: Spontaneously Combustible (UN 2008, UN 2009, UN 1932)
Zirconium Analytical Methods
For occupational chemical analysis use NIOSH: Elements (ICP), 7300.
Zirconium Specification
Handling: Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation. Wash thoroughly after handling. Wash hands before eating. Keep container tightly closed. Use with adequate ventilation. Minimize dust generation and accumulation.
Storage: Store in a cool, dry, well-ventilated area away from incompatible substances. Store in a tightly closed container.
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
 F,
F,  C
C








































































































![]()
 F,
F, Xi
Xi