-
Name
1,1-Dimethylhydrazine
- EINECS 200-316-0
- CAS No. 57-14-7
- Article Data64
- CAS DataBase
- Density 0.829 g/cm3
- Solubility miscible with water, ethanol, and kerosene
- Melting Point -57.2 °C
- Formula C2H8N2
- Boiling Point 63.9 °C at 760 mmHg
- Molecular Weight 60.0989
- Flash Point 1.1 °C
- Transport Information UN 1163
- Appearance clear colorless liquid with an ammonia-like odor
- Safety 53-45-61
- Risk Codes 45-11-23/25-34-51/53
-
Molecular Structure
-
Hazard Symbols
 F,
F,  T,
T,  N
N
- Synonyms 1,1-Dimethylhydrazine;Dimazin;Dimazine;Heptyl;N,N-Dimethylhydrazine;NSC 60517;UDMH;Unsymmetrical dimethylhydrazine;as-Dimethylhydrazine;gem-Dimethylhydrazine;u-Dimethylhydrazine;unsym-Dimethylhydrazine;
- PSA 29.26000
- LogP 0.12200
Synthetic route
-
-
127510-73-0
(5S,5aS,8aS)-5-(N',N'-Dimethyl-hydrazino)-1,3,7-trimethyl-5,5a,8a,9-tetrahydro-1H-pyrrolo[3,4-g]quinazoline-2,4,6,8-tetraone

-
A
-
127510-75-2
1,3,7-Trimethyl-8a,9-dihydro-1H-pyrrolo[3,4-g]quinazoline-2,4,6,8-tetraone

-
B
-
57-14-7
1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol | A 92% B n/a |

-
-
57-14-7
1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| With (η5-C5Me5)Rh(2-pyridylphenyl)H; hydrogen In tetrahydrofuran at 23℃; under 3040.2 Torr; for 120h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 87% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; formaldehyd; paraformaldehyde; platinum | 78% |


| Conditions | Yield |
|---|---|
| With titanium(III) chloride In water for 1h; Ambient temperature; | 70% |
| With lithium aluminium tetrahydride; diethyl ether | |
| With sodium hydroxide; aluminium |

-
-
57-14-7
1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| With cyclopentadienylchromiumtricarbonyl hydride; hydrogen In tetrahydrofuran at 23℃; under 3040.2 Torr; for 120h; Catalytic behavior; Reagent/catalyst; Inert atmosphere; Schlenk technique; | 15% |

| Conditions | Yield |
|---|---|
| With ammonia | |
| With ammonia; dimethyl amine at 35℃; under 9000.72 Torr; for 0.833333h; Product distribution; Further Variations:; educt/reagent ratios; | |
| With sodium amide In kerosene | |
| With tri-n-propylamine | |
| With ammonium hydroxide at 25℃; pH=13; Kinetics; |

| Conditions | Yield |
|---|---|
| With acetic acid; zinc | |
| With sulfuric acid durch elektrolytische Reduktion an einer verzinnten Kupferkathode; |
-
-
13750-17-9
N,N-dimethylcarbamoyl azide

-
-
57-14-7
1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| With xylene und Erhitzen des in Xylol unloeslichen Anteils des Reaktionsprodukts mit konz. Salzsaeure im Autoklaven auf 150grad; |

| Conditions | Yield |
|---|---|
| at 170 - 250℃; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; chloroamine In water at 25℃; Rate constant; Kinetics; variation of temperature, effect of pH and concentration of reagents; | |
| With chloroamine |

| Conditions | Yield |
|---|---|
| With water; hydrazine hydrate |
-
-
20241-01-4
1-(2'-nitrophenyl)-3,3-dimethyltriazene

-
A
-
57-14-7
1,1-dimethylhydrazine

-
B
-
95-54-5
1,2-diamino-benzene

| Conditions | Yield |
|---|---|
| With Britton-Robinson buffer In methanol Product distribution; Mechanism; pH dependence, polarographic reduction; |
-
-
60250-44-4
3-Amino-1,1-dimethyl-4,5-dihydro-1H-pyrazol-1-ium; iodide

-
-
57-14-7
1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 3.5h; Heating; Yield given; |

| Conditions | Yield |
|---|---|
| at 89.9 - 110.8℃; Rate constant; |

-
-
13166-44-4
dimethyltriazanium chloride

-
A
-
34557-54-5
methane

-
B
-
593-51-1
methylamine hydrochloride

-
C
-
57-14-7
1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| at 94.3 - 112.8℃; Rate constant; also heat evolution rates at 112.8 deg C in aqueous solutions and 0.1 N NaOH solution; |


| Conditions | Yield |
|---|---|
| With chloroamine at 18.9 - 31.9℃; Kinetics; Rate constant; pH 8 - pH 13; ΔH(excit), ΔS(excit), reactions under var. conditions; |
-
-
124-40-3
dimethyl amine

-
A
-
1585-74-6
dimethylchloroamine

-
B
-
2035-89-4
N-methyl-N-(methyleneamino)methanamine

-
C
-
35337-56-5
1,1-dimethyldiazene

-
D
-
57-14-7
1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| With chloroamine at 24.9℃; Mechanism; Rate constant; Kinetics; ΔH; ΔS; effect of pH; |

-
-
1585-74-6
dimethylchloroamine

-
-
57-13-6
urea

-
A
-
6415-12-9
tetramethylhydrazine

-
B
-
124-40-3
dimethyl amine

-
C
-
57-14-7
1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; tert-butyl alcohol for 8.25h; Ambient temperature; | A 17.2 % Spectr. B 4.3 % Spectr. C 2.4 % Spectr. |
| With potassium hydroxide In methanol; tert-butyl alcohol for 8.25h; Product distribution; Mechanism; Ambient temperature; | A 17.2 % Spectr. B 4.3 % Spectr. C 2.4 % Spectr. |

-
-
76093-78-2
1-amino-1,4,4-trimethylpiperidinium mesitylenesulfonate

-
-
74-88-4
methyl iodide

-
A
-
1112-35-2
3,3-dimethyl-penta-1,4-diene

-
B
-
57-14-7
1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tert-butyl alcohol for 24h; Product distribution; Heating; |

-
-
598-50-5
N-Methylurea

-
A
-
67-56-1
methanol

-
B
-
36214-48-9
formaldehyde monomethylhydrazone

-
C
-
57-14-7
1,1-dimethylhydrazine

-
D
-
60-34-4
methylhydrazine

| Conditions | Yield |
|---|---|
| With chloroamine In diethyl ether; water for 5h; Product distribution; Mechanism; other solvent, exposition; |


| Conditions | Yield |
|---|---|
| at 190℃; im Rohr; |

-
-
57-14-7
1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| under 150 Torr; bei der trocknen Destillation; |

| Conditions | Yield |
|---|---|
| bei der elektrolytischen Reduktion; |


| Conditions | Yield |
|---|---|
| at 27℃; Reaktion mit atomarem Wasserstoff; |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid In benzene Reflux; Dean-Stark; | 100% |
| With acetic acid In methanol Reflux; | 60% |
| In methanol at 20℃; | 31% |
-
-
1445-73-4
1-Methyl-4-piperidone

-
-
57-14-7
1,1-dimethylhydrazine

-
-
136415-15-1
1-methyl-4-piperidone dimethylhydrazone

| Conditions | Yield |
|---|---|
| With acetic acid In diethyl ether at 20℃; for 12h; | 100% |
-
-
1606-75-3
(2-oxoethyl)phosphonic acid diethyl ester

-
-
57-14-7
1,1-dimethylhydrazine

-
-
127056-04-6
[2-(dimethyl-hydrazino)-ethyl]-phosphonic acid diethyl ester

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 0℃; for 2h; | 100% |
| With magnesium sulfate In dichloromethane at 25℃; for 48h; |
-
-
19216-97-8
1,3-Bis<(phenylmethyl)thio>-2-propanone

-
-
57-14-7
1,1-dimethylhydrazine

-
-
122538-52-7
1,3-bis(benzylthio)acetone dimethylhydrazone

| Conditions | Yield |
|---|---|
| With sulfuric acid In ethanol for 7h; Heating; | 100% |
-
-
74181-34-3
2,2,-dimethyl-1,3-dioxan-5-one

-
-
57-14-7
1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| In benzene at 20℃; for 6h; Inert atmosphere; Reflux; | 100% |
| for 20h; Heating; | 87% |
-
-
115975-33-2
N,N-Dimethyl-2,4-bis(trifluoroacetyl)-1-naphthylamine

-
-
57-14-7
1,1-dimethylhydrazine

-
-
129602-61-5
N,N-dimethyl-N'-<2,4-bis(trifluoroacetyl)-1-naphthyl>hydrazine

| Conditions | Yield |
|---|---|
| In acetonitrile for 4h; Heating; | 100% |
-
-
111269-60-4
3,3,3-trifluoro-1-(p-tolyl)propane-1,2-dione

-
-
57-14-7
1,1-dimethylhydrazine

-
-
133593-18-7
2-(N',N'-Dimethyl-hydrazino)-3,3,3-trifluoro-2-hydroxy-1-p-tolyl-propan-1-one

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.166667h; | 100% |
-
-
133593-15-4
3,3,3-Trifluoro-1-o-tolyl-propane-1,2-dione

-
-
57-14-7
1,1-dimethylhydrazine

-
-
133593-22-3
2-(N',N'-Dimethyl-hydrazino)-3,3,3-trifluoro-2-hydroxy-1-o-tolyl-propan-1-one

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.166667h; | 100% |
-
-
111269-66-0
3,3,3-Trifluoro-1-(4-methoxy-phenyl)-propane-1,2-dione

-
-
57-14-7
1,1-dimethylhydrazine

-
-
133593-17-6
2-(N',N'-Dimethyl-hydrazino)-3,3,3-trifluoro-2-hydroxy-1-(4-methoxy-phenyl)-propan-1-one

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; for 0.166667h; | 100% |
-
-
57-14-7
1,1-dimethylhydrazine

-
-
529-20-4
2-methylphenyl aldehyde

-
-
59670-11-0
2-methylbenzaldehyde N,N-dimethylhydrazone

| Conditions | Yield |
|---|---|
| With magnesium sulfate In dichloromethane at 20℃; Inert atmosphere; | 100% |
| With magnesium sulfate In dichloromethane at 20℃; for 16h; | 66% |

| Conditions | Yield |
|---|---|
| iron(III) chloride In acetonitrile for 8h; Oxidation; condensation; Heating; | 100% |
| With iron(III) chloride In acetonitrile for 12h; Condensation; Heating; |
-
-
57-14-7
1,1-dimethylhydrazine

-
-
500102-99-8
1-[(2R,5R)-5-((E)-(R)-5-Benzyloxy-2,4-dimethyl-pent-3-enyl)-5-methyl-tetrahydro-furan-2-yl]-ethanone

| Conditions | Yield |
|---|---|
| With chloro-trimethyl-silane In dichloromethane at 0℃; | 100% |

| Conditions | Yield |
|---|---|
| at 0℃; | 100% |
-
-
106180-25-0
2-(1-hexynyl)-1-cyclopentenecarboxaldehyde

-
-
57-14-7
1,1-dimethylhydrazine

-
-
566190-89-4
N'-(2-hex-1-ynyl-cyclopent-1-enylmethylene)-N,N-dimethyl-hydrazine

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 16h; | 100% |
-
-
57-14-7
1,1-dimethylhydrazine

-
-
104429-45-0
(Z)-3-phenylnon-2-en-4-ynal

-
-
566190-70-3
N,N-dimethyl-N'-(3-phenyl-non-2-en-4-ynylidene)-hydrazine

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 16h; | 100% |
-
-
57-14-7
1,1-dimethylhydrazine

-
-
566190-80-5
2-ethyl-2-nonen-4-ynal

-
-
566190-81-6
N'-(2-ethyl-non-2-en-4-ynylidene)-N,N-dimethyl-hydrazine

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 16h; | 100% |
-
-
57-14-7
1,1-dimethylhydrazine

-
-
566190-78-1
2-nonen-4-ynal

-
-
566190-79-2
N,N-dimethyl-N'-non-2-en-4-ynylidene-hydrazine

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 16h; | 100% |
-
-
57-14-7
1,1-dimethylhydrazine

-
-
566190-83-8
2-(2-propenyl)-2-nonen-4-ynal

-
-
566190-86-1
N'-(2-allyl-non-2-en-4-ynylidene)-N,N-dimethyl-hydrazine

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 16h; | 100% |
-
-
57-14-7
1,1-dimethylhydrazine

-
-
566190-87-2
2-(hex-1-yn-1-yl)-1-cyclohexenecarboxaldehyde

-
-
566190-88-3
N'-(2-hex-1-ynyl-cyclohex-1-enylmethylene)-N,N-dimethyl-hydrazine

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 16h; | 100% |
-
-
1192-88-7
1-cyclohexene-1-carboxaldehyde

-
-
57-14-7
1,1-dimethylhydrazine

-
-
102268-18-8
1-cyclohexene-1-carboxaldehyde N,N-dimethylhydrazone

| Conditions | Yield |
|---|---|
| 100% |
-
-
57-14-7
1,1-dimethylhydrazine

-
-
650599-75-0
(2,7-di-tert-butyl-9,9-dimethyl)-9H-xanthene-4,5-dicarbaldehyde

| Conditions | Yield |
|---|---|
| In ethanol for 8h; Heating; | 100% |
| In ethanol Heating; |
-
-
896434-16-5
(3R,3aR,8R,8aR)-8-(tert-Butyl-dimethyl-silanyloxy)-3-methyl-octahydro-azulen-5-one

-
-
57-14-7
1,1-dimethylhydrazine

-
-
896434-17-6
N'-[8-(tert-butyl-dimethyl-silanyloxy)-3-methyl-octahydro-azulen-5-ylidene]-N,N-dimethyl-hydrazine

| Conditions | Yield |
|---|---|
| With acetic acid In ethanol at 20℃; for 1h; | 100% |
-
-
934278-96-3
2-methyl-4a,9a-dihydro-4H-1,3,5-trioxa-benzocyclohepten-7-one

-
-
57-14-7
1,1-dimethylhydrazine

-
-
934278-97-4
N,N-dimethyl-N'-(2-methyl-4a,9a-dihydro-4H-1,3,5-trioxa-benzocyclohepten-7-ylidene)-hydrazine

| Conditions | Yield |
|---|---|
| In benzene Heating; | 100% |

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; for 96h; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane (N2); stirring (-78°C, 0.08 h); warming to room temp., solvent removal (vac.), drying (vac., 0.5 h), pentane addn., stirring (0.2 h), filtn., washing (pentane), drying (vac.); | 100% |
-
-
4746-97-8
cyclohexanedione monoethylene ketal

-
-
57-14-7
1,1-dimethylhydrazine

-
-
1037668-50-0
2-[1,4-dioxaspiro[4.5 ]decan-8-ylidene]-1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| In toluene at 20℃; Dean-Stark; Reflux; | 100% |
| In toluene Dean-Stark; Reflux; | 100% |
| With trifluoroacetic acid In benzene Heating; | 86% |
| With toluene-4-sulfonic acid In benzene for 6h; Reflux; | |
| at 20℃; Neat (no solvent); |

| Conditions | Yield |
|---|---|
| Heating; | 100% |
-
-
3612-20-2
1-phenylmethyl-4-piperidone

-
-
57-14-7
1,1-dimethylhydrazine

-
-
1334292-35-1
1-benzyl-4-(2,2-dimethylhydrazono)piperidine

| Conditions | Yield |
|---|---|
| In toluene at 112℃; for 0.5h; Inert atmosphere; | 100% |
| In toluene for 2h; Dean-Stark; Reflux; | 100% |
-
-
2345-27-9
tetradecan-2-one

-
-
57-14-7
1,1-dimethylhydrazine

-
-
1356546-26-3
2-(tetradecan-2-ylidene)-1,1-dimethylhydrazine

| Conditions | Yield |
|---|---|
| In dichloromethane Reflux; | 100% |
| With magnesium sulfate In dichloromethane Inert atmosphere; Reflux; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; Inert atmosphere; | 100% |
| With magnesium sulfate In dichloromethane at 20℃; for 12h; |
1,1-Dimethylhydrazine Consensus Reports
1,1-Dimethylhydrazine Standards and Recommendations
ACGIH TLV: TWA 0.01 ppm (skin), Confirmed Animal Carcinogen.
DFG MAK: Animal Carcinogen, Suspected Human Carcinogen
NIOSH REL: (Hydrazines) CL 0.15 mg/m3/2H
DOT Classification: 6.1; Label: Poison, Flammable Liquid, Corrosive
1,1-Dimethylhydrazine Specification
The IUPAC name of this chemical is 1,1-dimethylhydrazine. With the CAS registry number 57-14-7 and EINECS 200-316-0, it is also named as Dimethylhydrazine unsymmetrical. The classification codes are Mutation data; Reproductive Effect; Tumor data. It is clear colorless liquid with an ammonia-like odor which is miscible with water, ethanol, and kerosene. It turns yellowish on exposure to air and absorbs oxygen and carbon dioxide. Moreover, this chemical is a powerful reducing agent and can exhibit high acute toxicity as a result of exposure by all routes. When heated to decomposition it emits highly toxic fumes of NOx. In addition, 1,1-Dimethylhydrazine should be sealed in the container and stored in the cool and dry place which must be away from oxidant and acids.
The other characteristics of this product can be summarized as: (1)ACD/LogP: -1.28; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -3.85; (4)ACD/LogD (pH 7.4): -2.16; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 2; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 6.48 Å2; (13)Index of Refraction: 1.426; (14)Molar Refractivity: 18.57 cm3; (15)Molar Volume: 72.4 cm3; (16)Polarizability: 7.36×10-24 cm3; (17)Surface Tension: 27.8 dyne/cm; (18)Density: 0.829 g/cm3; (19)Flash Point: 1.1 °C; (20)Enthalpy of Vaporization: 30.6 kJ/mol; (21)Boiling Point: 63.9 °C at 760 mmHg; (22)Vapour Pressure: 168 mmHg at 25°C.
Preparation of 1,1-Dimethylhydrazine: It can be obtained by the reaction of ammonia, ammonium chloride and dimethylamine. First, we can get chloro amine by ammonia and sodium hypochlorite. Secondly, the reaction of chloro amine and dimethylamine aqueous solution can generate UDMH solution. Finally, after a series of treatment, we can get the product.
NH3+NaOCl→NH2Cl+NaOH
NH2Cl+HN(CH3)3+NaOH→(CH3)2NNH2+NaCl+H2O
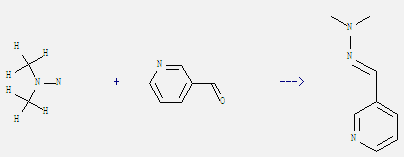
Uses of 1,1-Dimethylhydrazine: It is often used in hypergolic rocket fuels as a bipropellant in combination with the oxidizer nitrogen tetroxide and less frequently with IRFNA (red fuming nitric acid) or liquid oxygen. It is also used in the production of plant growth regulator. What's more, this chemical is used as fuel additive and used in organic synthesis. In addition, it can react with pyridine-3-carbaldehyde to get pyridine-3-carbaldehyde-dimethylhydrazone. This reaction needs solvent ethanol by heating on a steam bath. The reaction time is 30 min. The yield is 77%.
When you are using this chemical, please be cautious about it as the following:
It is highly flammable and can cause burns and cancer. What's more, it is toxic by inhalation and if swallowed. And it is also toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.) So it should avoid exposure - obtain special instructions before use and avoid release to the environment. Refer to special instructions / safety data sheets.
People can use the following data to convert to the molecule structure.
1. SMILES:N(N)(C)C
2. InChI:InChI=1/C2H8N2/c1-4(2)3/h3H2,1-2H3
3. InChIKey:RHUYHJGZWVXEHW-UHFFFAOYAL
The following are the toxicity data which has been tested.
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| cat | LDLo | intraperitoneal | 30mg/kg (30mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) VASCULAR: REGIONAL OR GENERAL ARTERIOLAR OR VENOUS DILATION LUNGS, THORAX, OR RESPIRATION: CYANOSIS | European Journal of Toxicology and Environmental Hygiene. Vol. 7, Pg. 238, 1974. |
| dog | LC50 | inhalation | 3580ppm/15M (3580ppm) | GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" BEHAVIORAL: TREMOR BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | American Industrial Hygiene Association Journal. Vol. 24, Pg. 137, 1963. |
| dog | LD50 | intraperitoneal | 60mg/kg (60mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES GASTROINTESTINAL: NAUSEA OR VOMITING | National Technical Information Service. Vol. AD292-689, |
| dog | LD50 | intravenous | 60mg/kg (60mg/kg) | Medycyna Pracy. Industrial Medicine. Vol. 24, Pg. 71, 1973. | |
| dog | LDLo | skin | 301mg/kg (301mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Toxicology and Applied Pharmacology. Vol. 18, Pg. 649, 1971. |
| guinea pig | LC50 | inhalation | 100mg/m3/4H (100mg/m3) | Toksikologicheskii Vestnik. Vol. (6), Pg. 39, 1999. | |
| guinea pig | LD50 | skin | 1329mg/kg (1329mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | U.S. Army Chemical Warfare Laboratories. Vol. CWL, |
| hamster | LC50 | inhalation | 392ppm/4H (392ppm) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | AMA Archives of Industrial Health. Vol. 12, Pg. 609, 1955. |
| hamster | LD50 | subcutaneous | 325mg/kg (325mg/kg) | Cancer Letters Vol. 35, Pg. 303, 1987. | |
| human | TDLo | unreported | 10mg/kg (10mg/kg) | BEHAVIORAL: HEADACHE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES LIVER: LIVER FUNCTION TESTS IMPAIRED | "Spravochnik po Toksikologii i Gigienicheskim Normativam Vol. -, Pg. 71, 1999. |
| monkey | LD50 | intraperitoneal | 60mg/kg (60mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD GASTROINTESTINAL: NAUSEA OR VOMITING | National Technical Information Service. Vol. AD292-689, |
| mouse | LC50 | inhalation | 172ppm/4H (172ppm) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA | AMA Archives of Industrial Health. Vol. 12, Pg. 609, 1955. |
| mouse | LD50 | intraperitoneal | 113mg/kg (113mg/kg) | SENSE ORGANS AND SPECIAL SENSES: MIOSIS (PUPILLARY CONSTRICTION): EYE SKIN AND APPENDAGES (SKIN): HAIR: OTHER BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Proceedings of the Society for Experimental Biology and Medicine. Vol. 124, Pg. 172, 1967. |
| mouse | LD50 | intravenous | 250mg/kg (250mg/kg) | Medycyna Pracy. Industrial Medicine. Vol. 24, Pg. 71, 1973. | |
| mouse | LD50 | oral | 155mg/kg (155mg/kg) | BRAIN AND COVERINGS: RECORDINGS FROM SPECIFIC AREAS OF CNS KIDNEY, URETER, AND BLADDER: "CHANGES IN TUBULES (INCLUDING ACUTE RENAL FAILURE, ACUTE TUBULAR NECROSIS)" BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | "Spravochnik po Toksikologii i Gigienicheskim Normativam Vol. -, Pg. 71, 1999. |
| rabbit | LD50 | skin | 1060mg/kg (1060mg/kg) | "Documentation of the Threshold Limit Values and Biological Exposure Indices," 5th ed., Cincinnati, OH, American Conference of Governmental Industrial Hygienists, Inc., 1986Vol. 5, Pg. 210.1(89), 1986. | |
| rat | LC50 | inhalation | 252ppm/4H (252ppm) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | AMA Archives of Industrial Health. Vol. 12, Pg. 609, 1955. |
| rat | LD50 | intraperitoneal | 102mg/kg (102mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Toxicology and Applied Pharmacology. Vol. 6, Pg. 371, 1964. |
| rat | LD50 | intravenous | 119mg/kg (119mg/kg) | Medycyna Pracy. Industrial Medicine. Vol. 24, Pg. 71, 1973. | |
| rat | LD50 | oral | 122mg/kg (122mg/kg) | Medycyna Pracy. Industrial Medicine. Vol. 24, Pg. 71, 1973. | |
| rat | LD50 | skin | 770mg/kg (770mg/kg) | BRAIN AND COVERINGS: RECORDINGS FROM SPECIFIC AREAS OF CNS BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BLOOD: CHANGES IN BONE MARROW NOT INCLUDED ABOVE | "Spravochnik po Toksikologii i Gigienicheskim Normativam Vol. -, Pg. 71, 1999. |
| rat | LDLo | intracrebral | 27mg/kg (27mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Biochemical Pharmacology. Vol. 14, Pg. 1901, 1965. |
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 5714-73-8
- 57149-07-2
- 5715-02-6
- 5715-23-1
- 57153-17-0
- 57153-43-2
- 57153-55-6
- 571-55-1
- 57156-91-9
- 571-57-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View