-
Name
1,3-Dihydroxyadamantane
- EINECS
- CAS No. 5001-18-3
- Article Data95
- CAS DataBase
- Density 1.368 g/cm3
- Solubility Soluble in organic solvents,insoluble in water.
- Melting Point ~260 °C
- Formula C10H16O2
- Boiling Point 320.4 °C at 760 mmHg
- Molecular Weight 168.236
- Flash Point 158.5 °C
- Transport Information
- Appearance white crystal
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1,3-Adamantanediol(6CI,7CI,8CI);1,3-Dihydroxy admantane;Tricyclo[3.3.1.13,7]decane-1,3-diol;
- PSA 40.46000
- LogP 1.06240
Synthetic route
-
-
16104-50-0
1,3-dichloroadamantane

-
-
5001-18-3
1,3-adamantandiol

| Conditions | Yield |
|---|---|
| With water; sodium acetate In DMF (N,N-dimethyl-formamide) at 150℃; under 2175.22 Torr; for 18h; Product distribution / selectivity; | 96% |
| With water; sodium acetate In ISOPROPYLAMIDE at 150℃; under 2175.22 Torr; for 18h; Product distribution / selectivity; | 96% |
| With lithium acetate; water In DMF (N,N-dimethyl-formamide) at 150℃; under 2175.22 Torr; for 18h; Product distribution / selectivity; | 96% |

| Conditions | Yield |
|---|---|
| Stage #1: 1-adamanthanol With sulfuric acid; acetic anhydride at 14 - 16℃; for 0.5h; Large scale; Stage #2: With nitric acid at 83 - 102℃; Temperature; Large scale; Further stages; | 95.1% |
| With ruthenium trichloride; sodium periodate In tetrachloromethane; water; acetonitrile at 60℃; for 8h; | 92% |
| With Bromotrichloromethane; water; molybdenum hexacarbonyl at 150℃; for 6h; Inert atmosphere; regioselective reaction; | 86% |
-
-
84868-17-7
p-Toluolsulfonsaeure-(3-chlor-1-adamantyl)ester

-
A
-
5001-18-3
1,3-adamantandiol

-
B
-
772-26-9
1-chloro-3-hydroxyadamantane

| Conditions | Yield |
|---|---|
| With 1,4-dioxane; water; triethylamine solvolysis; | A 5% B 94% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-dibromoadamantane With sodium formate In formic acid at 115 - 120℃; Large scale; Stage #2: With water at 80℃; Large scale; | 91.7% |
| With water In acetone for 3h; Reflux; | 68.5% |
| With silver nitrate In 1,4-dioxane | |
| With acetone for 3h; Reflux; |
-
-
281-23-2
adamantane

-
-
74087-85-7
3,3-dimethyldioxirane

-
A
-
700-58-3
2-Adamantanone

-
B
-
768-95-6
1-adamanthanol

-
C
-
700-57-2
1-adamantanol

-
D
-
5001-18-3
1,3-adamantandiol

-
E
-
22635-62-7
1-acetyloxyadamantane

| Conditions | Yield |
|---|---|
| In acetone at 20℃; for 7.6h; Rate constant; Product distribution; Kinetics; | A 1.5% B 91.5% C 1% D 4.5% E 1.5% |

-
-
281-23-2
adamantane

-
A
-
700-58-3
2-Adamantanone

-
B
-
768-95-6
1-adamanthanol

-
C
-
5001-18-3
1,3-adamantandiol

| Conditions | Yield |
|---|---|
| With methyltrifluoromethyldioxirane In dichloromethane at -22℃; for 0.0166667h; | A n/a B n/a C 86% |
| With 6-chloro-4-trifluoromethyl-1,2,3-benzoxathiazine-2,2-dioxide; urea hydrogen peroxide adduct; bis[3,5-bis(trifluoromethyl)diphenyl] diselenide In 1,2-dichloro-ethane at 22℃; for 95h; | A n/a B 80% C 5% |
| With tetrabutylammomium bromide; oxygen; N-hydroxyphthalimide In water at 80℃; under 760 Torr; for 6h; Product distribution; further additives, solvents; | A 12% B 60% C 23% |


| Conditions | Yield |
|---|---|
| With methyltrifluoromethyldioxirane In dichloromethane at -20℃; for 0.666667h; | A 4 % Chromat. B 86% |
| With acetic anhydride; periodic acid; chromoyl diacetate In dichloromethane; acetonitrile at -40 - 0℃; for 2h; | A 86% B n/a |
| With 2,2,2-trifluoro-1-(4-fluorophenyl)ethanone; dihydrogen peroxide; acetic acid In 1,2-dichloro-ethane at 70℃; for 72h; Catalytic behavior; Reagent/catalyst; chemoselective reaction; | A 81% B 7% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; cobalt(II) acetate In dichloromethane at 0℃; for 3h; Reagent/catalyst; | 83.4% |
| With chromium(VI) oxide; sulfuric acid; acetic acid at 80℃; for 2h; | 71% |
| With N-hydroxyphthalimide; oxygen; cobalt acetylacetonate In acetic acid at 75℃; under 760 Torr; for 6h; | 98 % Turnov. |
-
-
84868-18-8
p-Toluolsulfonsaeure-(3-brom-1-adamantyl)ester

-
A
-
5001-18-3
1,3-adamantandiol

-
B
-
17933-29-8
7-methylenebicyclo[3.3.1]nonan-3-one

| Conditions | Yield |
|---|---|
| With 1,4-dioxane; water; triethylamine solvolysis; | A 75% B 2% |
-
-
281-23-2
adamantane

-
-
111-27-3
hexan-1-ol

-
A
-
5001-18-3
1,3-adamantandiol

-
B
-
99181-50-7
adamantane-1,3,5-triol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; sodium hypochlorite | A n/a B 70% |


-
-
281-23-2
adamantane

-
-
111-27-3
hexan-1-ol

-
A
-
5001-18-3
1,3-adamantandiol

-
B
-
99181-50-7
adamantane-1,3,5-triol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hydroxide; sodium hypochlorite In water; ethyl acetate | A 70% B n/a |

-
-
281-23-2
adamantane

-
A
-
700-58-3
2-Adamantanone

-
B
-
768-95-6
1-adamanthanol

-
C
-
5001-18-3
1,3-adamantandiol

-
D
-
772-26-9
1-chloro-3-hydroxyadamantane

| Conditions | Yield |
|---|---|
| With tetrachloromethane; water; molybdenum hexacarbonyl at 140℃; for 13h; Inert atmosphere; | A 10% B 70% C n/a D n/a |

-
-
39269-10-8
adamantane-1,3-dicarboxylic acid

-
-
5001-18-3
1,3-adamantandiol

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; sulfuric acid at 43 - 47℃; | 67% |

| Conditions | Yield |
|---|---|
| A n/a B 65% | |
| With hydrogenchloride | A n/a B 61% |
| A n/a B 52% | |
| With methyltrifluoromethyldioxirane In dichloromethane at -20℃; for 2h; | A 8 % Chromat. B 90 % Chromat. |
| With C25H22FeN7O3(2+)*2ClO4(1-); dihydrogen peroxide In acetonitrile at 25℃; for 5h; | A 18.9 %Chromat. B 27.2 %Chromat. |
-
-
24569-89-9
1,3-dehydroadamantane

-
A
-
5001-18-3
1,3-adamantandiol

-
B
-
772-26-9
1-chloro-3-hydroxyadamantane

| Conditions | Yield |
|---|---|
| With chromyl chloride In tetrachloromethane; dichloromethane at 0℃; | A 24% B 64% |
-
-
41826-65-7
adamantane-1,3-dicarboxylic acid diethyl ester

-
-
5001-18-3
1,3-adamantandiol

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; sulfuric acid at 25 - 30℃; | 63% |

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; N-hydroxyphthalimide; oxygen; cobalt acetylacetonate In acetic acid at 60℃; under 760.051 Torr; for 30h; Reagent/catalyst; Concentration; Temperature; Time; chemoselective reaction; | A 39% B 52% |
-
-
281-23-2
adamantane

-
A
-
700-58-3
2-Adamantanone

-
B
-
768-95-6
1-adamanthanol

-
C
-
700-57-2
1-adamantanol

-
D
-
5001-18-3
1,3-adamantandiol

| Conditions | Yield |
|---|---|
| With BaFeO(2.8-x); oxygen at 89.84℃; under 750.075 Torr; for 96h; Catalytic behavior; | A 5% B 48% C 3% D 11% |
| With oxygen; propionic acid; bis(acetylacetonate)oxovanadium at 120℃; under 760.051 Torr; for 6h; Product distribution / selectivity; | A 5.5% B 31.6% C 3.9% D 7.9% |
| With methanesulfonic acid; oxygen; propionic acid; bis(acetylacetonate)oxovanadium at 100℃; under 760.051 Torr; for 6h; Product distribution / selectivity; | A 4.2% B 25.1% C 3.9% D 2.4% |

-
-
24569-89-9
1,3-dehydroadamantane

-
A
-
5001-18-3
1,3-adamantandiol

-
B
-
17933-29-8
7-methylenebicyclo[3.3.1]nonan-3-one

| Conditions | Yield |
|---|---|
| With 3,3-dimethyldioxirane In dichloromethane; acetone for 20h; | A 8% B 46% |
-
-
281-23-2
adamantane

-
A
-
768-95-6
1-adamanthanol

-
B
-
5001-18-3
1,3-adamantandiol

-
C
-
700-57-2
2-hydroxytricyclo<3.3.1.13,7>decane

-
D
-
20098-16-2, 73346-81-3, 73346-82-4
tricyclo<3.3.1.13,7>decane-1-4ax-diol

| Conditions | Yield |
|---|---|
| With Absidia glauca (IMI239693) In N,N-dimethyl-formamide at 23℃; for 260h; Product distribution; var. fungi of the genus Absidia and times; | A 24% B 4% C 3% D 8% |

-
-
281-23-2
adamantane

-
-
64-19-7
acetic acid

-
A
-
700-58-3
2-Adamantanone

-
B
-
19066-22-9
2-acetoxyadamantane

-
C
-
768-95-6
1-adamanthanol

-
D
-
700-57-2
1-adamantanol

-
E
-
5001-18-3
1,3-adamantandiol

-
F
-
22635-62-7
1-acetyloxyadamantane

-
G
-
56137-59-8
1-hydroxy-3-acetoxyadamantane

-
H
-
29817-47-8
1,3-dihydroxyadamantane diacetate

| Conditions | Yield |
|---|---|
| With oxygen; vanadium trisacetylacetonate at 120℃; under 760.051 Torr; for 6h; Product distribution / selectivity; | A 3.8% B n/a C 22.3% D 3% E 3.8% F n/a G n/a H n/a |
| With oxygen; bis(acetylacetonate)oxovanadium at 120℃; under 760.051 Torr; for 6h; Product distribution / selectivity; | A 3.1% B n/a C 18% D 2.8% E 1.7% F n/a G n/a H n/a |
| With oxygen; ammonium metavanadate at 120℃; under 760.051 Torr; for 6h; Product distribution / selectivity; | A 1.5% B n/a C 10.1% D 1.5% E 0.6% F n/a G n/a H n/a |


| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 23℃; for 150h; culture of Absidia cylindrospora (I.M.I. 342950); | A 20% B 14% |
-
-
880-52-4
N-(1-adamantyl)acetamide

-
A
-
5001-18-3
1,3-adamantandiol

-
B
-
702-82-9
3-aminoadamantan-1-ol

-
C
-
778-10-9
N-(3-hydroxyadamantan-1-yl)acetamide

| Conditions | Yield |
|---|---|
| Stage #1: N-(1-adamantyl)acetamide With nitric acid Stage #2: With acetic acid; urea In water | A 11% B n/a C 13% |

-
-
281-23-2
adamantane

-
A
-
768-95-6
1-adamanthanol

-
B
-
5001-18-3
1,3-adamantandiol

-
C
-
99181-50-7
adamantane-1,3,5-triol

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium hypochlorite; ruthenium trichloride In water; ethyl acetate at 46℃; for 0.6 - 6.66667h; pH=4 - 8.5; | A 5% B 4% C 0.3% |
| With chromium(VI) oxide; acetic acid In water at 100℃; Temperature; Time; |

-
-
281-23-2
adamantane

-
-
115464-59-0
methyltrifluoromethyldioxirane

-
A
-
768-95-6
1-adamanthanol

-
B
-
5001-18-3
1,3-adamantandiol

| Conditions | Yield |
|---|---|
| In dichloromethane at -12.4℃; for 2h; Rate constant; Mechanism; Product distribution; | A 38 % Chromat. B 60 % Chromat. |

-
-
281-23-2
adamantane

-
-
74087-85-7
3,3-dimethyldioxirane

-
A
-
768-95-6
1-adamanthanol

-
B
-
5001-18-3
1,3-adamantandiol

| Conditions | Yield |
|---|---|
| In dichloromethane; acetone at -12.4℃; for 2h; Rate constant; |


| Conditions | Yield |
|---|---|
| With difluoroether |

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; Inert atmosphere; Cooling with ice; | 97% |
-
-
5001-18-3
1,3-adamantandiol

-
-
814-68-6
acryloyl chloride

-
-
216581-76-9
acrylic acid 3-hydroxy-1-adamantyl

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; Cooling with ice; | 90.9% |
-
-
5001-18-3
1,3-adamantandiol

| Conditions | Yield |
|---|---|
| With 1H-imidazole; dmap In tetrahydrofuran for 2h; Inert atmosphere; | 90% |
-
-
5001-18-3
1,3-adamantandiol

-
-
17933-29-8
7-methylenebicyclo[3.3.1]nonan-3-one

| Conditions | Yield |
|---|---|
| With pyridine; p-toluenesulfonyl chloride In benzene at 70℃; for 12h; | 88% |
| With p-toluenesulfonyl chloride In pyridine; benzene at 75℃; for 12h; | 81% |
| With pyridine; p-toluenesulfonyl chloride In benzene | |
| With pyridine; p-toluenesulfonyl chloride In benzene at 75℃; for 40h; Grob Fragmentation; Inert atmosphere; |
-
-
307-24-4
undecafluorohexanoic acid

-
-
5001-18-3
1,3-adamantandiol

-
-
1005454-12-5
3-hydroxy-1-adamantyl perfluorohexanoate

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 4h; Heating / reflux; | 88% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-adamantandiol With sulfuric acid In water at 20℃; for 1h; Stage #2: formic acid In water at 0 - 20℃; for 4h; Stage #3: With sodium hydroxide; sulfuric acid more than 3 stages; | 85% |
| With sulfuric acid at 0 - 20℃; for 12h; Koch-Haaf Carboxylation; | 79.5% |
| With sulfuric acid at 20℃; for 12h; Cooling with ice; | 79.5% |

| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid In 1,2-dimethoxyethane at 90℃; for 6h; | 85% |
-
-
91-10-1
1,3-dimethoxy-2-hydroxy-benzene

-
-
5001-18-3
1,3-adamantandiol

-
-
1166222-89-4
1,3-bis(4-hydroxy-3,5-dimethoxy-phenyl)-adamantane

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; trifluoroacetic acid In 1,2-dichloro-ethane | 85% |

| Conditions | Yield |
|---|---|
| With indium(III) triflate In 1,2-dichloro-ethane for 3h; Reflux; | 83% |

| Conditions | Yield |
|---|---|
| With pyridine at 60 - 95℃; | 83% |

| Conditions | Yield |
|---|---|
| 1.) 0 deg C, 15 min, 2.) r.t., 1 h; reflux, 2 h; | 82% |

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In ethylene dibromide for 48h; Inert atmosphere; | 82% |
-
-
5001-18-3
1,3-adamantandiol

-
-
95-48-7
ortho-cresol

-
-
582311-10-2
1,3-bis(3-methyl-4-hydroxyphenyl)adamantane

| Conditions | Yield |
|---|---|
| toluene-4-sulfonic acid at 90℃; for 10h; | 80% |
| With sodium hydroxide; phosphoric acid; p-toluenesulfonic acid monohydrate In nitrogen; water; toluene |

-
-
5001-18-3
1,3-adamantandiol

-
-
99181-50-7
adamantane-1,3,5-triol

| Conditions | Yield |
|---|---|
| With acetic acid | 80% |
-
-
5001-18-3
1,3-adamantandiol

| Conditions | Yield |
|---|---|
| With pyridine; p-toluenesulfonyl chloride In benzene at 75℃; for 12h; | 80% |
| With dmap; p-toluenesulfonyl chloride In toluene at 130℃; for 1.5h; Microwave irradiation; | 77% |
| With pyridine; p-toluenesulfonyl chloride at 70℃; for 6h; | 70.4% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid for 20h; Reflux; | 76% |
| With methanesulfonic acid In melt at 80 - 90℃; for 7h; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| With sodium carbonate; bis(1,5-cyclooctadiene)diiridium(I) dichloride In toluene at 100℃; for 6h; | 75% |
-
-
75-89-8
2,2,2-trifluoroethanol

-
-
5001-18-3
1,3-adamantandiol

-
-
1058167-51-3
3-(2,2,2-trifluoroethoxy)adamantan-1-ol

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid at 40℃; for 3h; | 75% |
| With trifluorormethanesulfonic acid at 40℃; for 2.5h; | 75% |
-
-
5001-18-3
1,3-adamantandiol

-
-
120-80-9
benzene-1,2-diol

-
-
128455-01-6
1,3-bis(3,4-dihydroxyphenyl)adamantane

| Conditions | Yield |
|---|---|
| With methanesulfonic acid at 80℃; for 3h; Inert atmosphere; | 72% |
| With methanesulfonic acid at 80℃; for 3h; | 20% |
1,3-Dihydroxyadamantane Chemical Properties
Molecular Structure: 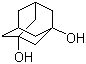
Molecular Formula: C10H16O2
Molecular Weight: 168.23
CAS NO: 5001-18-3
H bond acceptors: 2
H bond donors: 2
Freely Rotating Bonds: 2
Polar Surface Area: 18.46 Å2
Index of Refraction: 1.656
Molar Refractivity: 45.2 cm3
Molar Volume: 122.9 cm3
Surface Tension: 71.5 dyne/cm
Density: 1.368 g/cm3
Flash Point: 158.5 °C
Enthalpy of Vaporization: 65.15 kJ/mol
Boiling Point: 320.4 °C at 760 mmHg
Vapour Pressure: 2.59E-05 mmHg at 25°C
Melting Point: ~260 °C
Appearance: white crystal
SMILES: OC13CC2CC(O)(CC(C1)C2)C3
InChI: InChI=1/C10H16O2/c11-9-2-7-1-8(4-9)5-10(12,3-7)6-9/h7-8,11-12H,1-6H2
InChIKey: MOLCWHCSXCKHAP-UHFFFAOYAO
Std. InChI: InChI=1S/C10H16O2/c11-9-2-7-1-8(4-9)5-10(12,3-7)6-9/h7-8,11-12H,1-6H2
Std. InChIKey: MOLCWHCSXCKHAP-UHFFFAOYSA-N
Product Categories of 1,3-Dihydroxyadamantane (CAS NO.5001-18-3): Pharmaceutical Intermediates;Adamantane derivatives;Adamantanes
1,3-Dihydroxyadamantane Safety Profile
WGK Germany: 3
1,3-Dihydroxyadamantane Specification
The chemical synonyms of 1,3-Dihydroxyadamantane (CAS NO.5001-18-3): 1,3-AdamantandioL ; 1,3-DihydroxyadamantanE ; Adamantane-1,3-diol ; 1,3-Admantane-dimethanol ; 1,3-Admantane .
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 500128-99-4
- 5001-33-2
- 5001-44-5
- 500148-38-9
- 5001-51-4
- 500160-56-5
- 500167-99-7
- 500169-90-4
- 5001-72-9
- 5001-94-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View