-
Name
1,4-DIHYDROXYANTHRAQUINONE
- EINECS 201-368-7
- CAS No. 81-64-1
- Article Data141
- CAS DataBase
- Density 1.54 g/cm3
- Solubility <1 g/L (20 °C) in water
- Melting Point 198-199 °C(lit.)
- Formula C14H8O4
- Boiling Point 465.3 °C at 760 mmHg
- Molecular Weight 240.215
- Flash Point 249.3 °C
- Transport Information
- Appearance orange to red-brown crystalline powder
- Safety 22-24/25-36/37-26
- Risk Codes 36/37/38-43
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Anthraquinone,1,4-dihydroxy- (8CI);1,4-Dihydroxy-9,10-anthracenedione;1,4-Dihydroxy-9,10-anthraquinone;1,4-Dihydroxyanthraquinone;C.I. 58050;C.I.Solvent Orange 100;C.I. Solvent Orange 86;Chinizarin;Macrolex OrangeGG;NSC 15367;NSC 229036;NSC 40899;NSC 646569;Quinizarin;Quinizarine;Smoke Orange 18;Smoke Orange R;Solvent Orange 100;Solvent Orange 86;
- PSA 74.60000
- LogP 1.87320
Synthetic route
-
-
100073-85-6
N-(p-tolyl)-1,4-dihydroxy-9,10-anthraquinone 9-imine

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sulfuric acid at 20℃; for 1h; | 100% |

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With pyridine In chloroform for 48h; Ambient temperature; | 100% |
-
-
17648-03-2
9,10-Dihydroxy-2,3-dihydro-anthracene-1,4-dione

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With nitric acid; bentonite for 0.166667h; Product distribution; Further Variations:; Reagents; reaction times; types of irradiation; Oxidation; Irradiation; | 100% |
| With sodium hydroxide; oxygen |

| Conditions | Yield |
|---|---|
| With boron tribromide In dichloromethane at -78℃; for 0.166667h; | 100% |
-
-
33266-08-9
1,4-dibenzoyloxy-9,10-anthraquinone

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With water; trifluoroacetic acid at 72℃; for 0.0833333h; | 99% |

| Conditions | Yield |
|---|---|
| With water; trifluoroacetic acid at 72℃; for 0.0833333h; | 96% |
| Multi-step reaction with 2 steps 1: 4 percent / alkali-metal alcoholate / 70 °C 2: 91 percent / conc. aq. HCl / ethanol / 1 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: sodium isopropoxide / propan-2-ol / 0.5 h / 70 °C 2: 100 percent / conc. H2SO4 / 1 h / 20 °C View Scheme | |
| With lipase supported in poly(methylmethacrylate) beads; water In cyclohexane at 24.84℃; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With sulfuric acid; boric acid at 190 - 225℃; for 5h; | 94% |
| With fuming sulphuric acid; boric acid at 25℃; for 11h; Temperature; Solvent; Concentration; Reagent/catalyst; | 90% |
| With sulfuric acid; boric acid at 200℃; for 3.5h; | 85% |
-
-
98771-41-6
9,10-di(p-tolylamino)-1,4-anthraquinone

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With hydrogenchloride In ethanol for 1h; Heating; | 91% |
-
-
1709-63-3
anthracene-1,4,9,10-tetraone

-
-
107-15-3
ethylenediamine

-
A
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
B
-
20269-39-0
6-hydroxy-1,2,3,4-tetrahydronaphtho<2,3-f>quinoxaline-7,12-dione

| Conditions | Yield |
|---|---|
| In pyridine Ambient temperature; | A 1.2% B 90.7% |
| In pyridine at 30℃; for 6h; | A 1.2% B 90.7% |

-
-
1709-63-3
anthracene-1,4,9,10-tetraone

-
-
24454-89-5
2-acetoxy-1,3-butadiene

-
A
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
B
-
63229-39-0
2-acetoxy-1,4,4a,12a-tetrahydronaphthacene-5,6,11,12-tetrone

| Conditions | Yield |
|---|---|
| In benzene at 55℃; for 96h; | A n/a B 83% C n/a |
| In benzene at 55℃; for 96h; Title compound not separated from byproducts; |

-
-
475-38-7
5,8-Dihydroxy-1,4-naphthoquinone

-
-
123-73-9
crotonaldehyde

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With benzoic acid; L-proline In toluene at 50℃; for 5h; Inert atmosphere; Enzymatic reaction; | 82% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; boric acid for 5h; Irradiation; | 81% |
| With air; sulfuric acid Product distribution; Mechanism; Irradiation; without air (argon); |
-
-
84-65-1
9,10-phenanthrenequinone

-
A
-
605-32-3
2-hydroxy-9,10-anthraquinone

-
B
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sulfuric acid; boric acid for 5h; Product distribution; Mechanism; Irradiation; other time; | A n/a B 81% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; sodium chloride at 165℃; for 4h; Fridel-Crafts acylation; | 80% |
| With sulfuric acid; boric acid at 200℃; for 3.5h; | 55% |
| With aluminum (III) chloride; sodium chloride at 165 - 170℃; for 0.75h; Friedel-Crafts Acylation; | 44% |

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sodium trimethylsilanolate In tetrahydrofuran Substitution; stirring f the mixture at room temperature under a nitrogen atmosphere for 18 h; | 80% |
-
-
28736-42-7
1,4-difluoroanthracene-9,10-dione

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With methyl propargyl alcohol; potassium tert-butylate In dimethyl sulfoxide at 125℃; for 0.0333333h; microwave irradiation; | 78% |
| Multi-step reaction with 2 steps 1: 90 percent / NaOSi(CH3)3 / tetrahydrofuran / stirring f the mixture at room temperature under a nitrogen atmosphere for 18 h 2: 80 percent / NaOSi(CH3)3 / tetrahydrofuran / stirring f the mixture at room temperature under a nitrogen atmosphere for 18 h View Scheme |
-
-
88-95-9
Phthaloyl dichloride

-
-
150-78-7
1,4-dimethoxybezene

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With aluminium trichloride In nitrobenzene at 80℃; for 0.5h; | 72% |
-
-
29525-00-6
2,3-dichloro-1,4-dihydroxyanthracene-9,10-dione

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-dichloro-1,4-dihydroxyanthracene-9,10-dione With iron In acetic acid for 0.75h; Dehalogenation; reduction; Heating; Stage #2: With oxygen In sodium hydroxide pH=8 - 9; Oxidation; | 72% |
-
-
84-65-1
9,10-phenanthrenequinone

-
A
-
605-32-3
2-hydroxy-9,10-anthraquinone

-
B
-
129-43-1
1-hydroxyanthraquinone

-
C
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sulfuric acid at 25℃; Irradiation; | A 70% B n/a C n/a |
| With sulfuric acid at 25℃; Irradiation; prolonged irradiation; | A n/a B 55% C 6% |

-
-
106-48-9
4-chloro-phenol

-
-
88-99-3
benzene-1,2-dicarboxylic acid

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With sulfuric acid; boric acid at 230℃; for 0.166667h; Product distribution; Further Variations:; Temperatures; Reagents; reaction times; microwave powers; microwave irradiation; | 68.3% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride In nitrobenzene at 100℃; for 0.5h; | 65% |
| With aluminium trichloride; C6H4NO2 at 165℃; for 4h; Fridel-Crafts acylation; |
-
-
51287-54-8
3-(phenylsulfanyl)isobenzofuran-1(3H)-one

-
-
106-51-4
p-benzoquinone

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| Stage #1: 3-(phenylsulfanyl)isobenzofuran-1(3H)-one With lithium tert-butoxide In tetrahydrofuran at -60℃; for 0.416667h; Stage #2: p-benzoquinone In tetrahydrofuran at -60 - 20℃; Hauser annulation; Further stages.; | 56% |
-
-
82-34-8
1-nitroanthraquinone

-
A
-
129-43-1
1-hydroxyanthraquinone

-
B
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
C
-
72-48-0
1,2-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With potassium hydroxide; Cumene hydroperoxide In dimethyl sulfoxide at 15 - 25℃; for 10h; | A 55% B 13% C 32% |
| With Cumene hydroperoxide; potassium tert-butylate In N,N,N,N,N,N-hexamethylphosphoric triamide at 15 - 20℃; for 3h; | A 45% B 8% C 35% |

-
-
27613-27-0
3-oxo-1,3-dihydroisobenzofuran-1-carbonitrile

-
-
106-51-4
p-benzoquinone

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| Stage #1: 3-oxo-1,3-dihydroisobenzofuran-1-carbonitrile With lithium tert-butoxide In tetrahydrofuran at -60℃; for 0.416667h; Stage #2: p-benzoquinone In tetrahydrofuran at -60 - 20℃; Hauser annulation; Further stages.; | 52% |
-
-
67-56-1
methanol

-
-
41099-45-0
2,10-hydroxy-9-chloro-1,4-anthraquinone

-
A
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
B
-
7336-64-3
1-hydroxy-4-methoxyanthracene-9,10-dione

| Conditions | Yield |
|---|---|
| With perchloric acid for 12h; Heating; | A 36% B 49% |

-
-
38399-72-3
leuco-1,4-dihydroxyanthraquinone

-
-
15186-48-8
2,3-isopropylidene-glyceraldehyde

-
A
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
B
-
128885-71-2
dihydroxy-1,4 (hydroxy-3 propyl)-2 anthraquinone-9,10

-
C
-
111301-39-4
(-)-(S)<(dimethyl-2,2 dioxolanne-1,3 yl-4) methyl>-2 dihydroxy-1,4 anthraquinone-9,10

-
D
-
111301-40-7, 111331-26-1
<(dimethyl-2,2 dioxolanne-1,3 yl-4) hydroxymethyl>-2 dihydroxy-1,4 anthraquinone-9,10

| Conditions | Yield |
|---|---|
| With potassium hydroxide; sodium hydrogensulfite Product distribution; 1.) MeOH, 15 min, 20 deg C, 2.) MeOH, 12 h, 20 deg C, further temperatures; | A 33% B 4% C 22% D 37% |

-
-
79207-97-9
1,4-bis(prop-2'-enyloxy)-9,10-anthraquinone

-
A
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
B
-
79208-09-6
1,4-dihydroxy-2-(prop-2'-enyl)-9,10-anthraquinone

-
C
-
79208-01-8
2,3-diallyl-1,4-dihydroxyanthraquinone

-
D
-
79208-08-5
1-hydroxy-4-propanoyl-2-(prop-2'-enyl)anthraquinone

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-benzene for 25h; Heating; | A 5% B 32% C 14% D 18% |

-
-
14569-45-0
5,8-diacetoxy-1,4-naphthoquinone

-
-
108030-46-2
(E,E)-1-(N,N-dimethylamino)-4-nitro-1,3-butadiene

-
A
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
B
-
27573-16-6
5-nitro-1,4-dihydroxy-9,10-anthracenedione

-
C
-
117554-15-1
Acetic acid 4-hydroxy-5-nitro-9,10-dioxo-9,10-dihydro-anthracen-1-yl ester

-
D
-
117554-14-0
Acetic acid 4-hydroxy-8-nitro-9,10-dioxo-9,10-dihydro-anthracen-1-yl ester

| Conditions | Yield |
|---|---|
| In xylene for 24h; Ambient temperature; | A 4.2% B 30.2% C n/a D n/a |
| In xylene for 24h; Ambient temperature; Title compound not separated from byproducts; | A 4.2% B 30.2% C n/a D n/a |

-
-
108030-46-2
(E,E)-1-(N,N-dimethylamino)-4-nitro-1,3-butadiene

-
-
475-38-7
5,8-Dihydroxy-1,4-naphthoquinone

-
A
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
B
-
27573-16-6
5-nitro-1,4-dihydroxy-9,10-anthracenedione

-
C
-
117554-13-9
2-(dimethylamino)-5,8-dihydroxynaphthalene-1,4-dione

| Conditions | Yield |
|---|---|
| In xylene for 12h; Ambient temperature; | A 9.2% B 14.1% C 24.9% |

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
106-95-6
allyl bromide

-
-
79207-97-9
1,4-bis(prop-2'-enyloxy)-9,10-anthraquinone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 55℃; for 16h; | 100% |
| With potassium carbonate In acetone for 48h; Reflux; | 89% |
| With tetraethylammonium bromide; potassium carbonate; sodium hydroxide at 85℃; for 0.5h; Microwave irradiation; Sealed tube; | 85% |
| With potassium carbonate In acetone Heating; | 81% |
| With potassium carbonate In acetone Reflux; |
-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
77-78-1
dimethyl sulfate

-
-
6119-74-0
1,4-dimethoxyanthraquinone

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran for 480h; Ambient temperature; | 99% |
| With potassium carbonate In acetone for 4h; Reflux; | 96% |
| With potassium carbonate In acetone for 72h; Heating; | 95% |
-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
107-15-3
ethylenediamine

-
-
15077-66-4
6-hydroxy-1,2,3,4-tetrahydronaphtho[2,3-f]-quinoxaline-7,12-dione

| Conditions | Yield |
|---|---|
| With [bmim]PF6; copper dichloride at 20℃; for 2h; Product distribution; Further Variations:; Reagents; | 99% |
-
-
14285-68-8
decacarbonyldirhenium(0)

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
7128-64-5
Uvitex OB

-
-
918153-35-2
[(Re(CO)3)4(2,5-bis(5-tert-butyl-2-benzoxazolyl)thiophene)2(1,4-dihydroxy-9,10-anthraquinone(-2H))2]

| Conditions | Yield |
|---|---|
| In 1,2,5-trimethyl-benzene under reflux; | 99% |

-
-
34841-06-0
3-bromo-4-methoxybenzylaldehyde

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-dihydroxy-9,10-anthracenedione With potassium hydroxide In methanol; water for 0.5h; Inert atmosphere; Cooling with ice; Stage #2: 3-bromo-4-methoxybenzylaldehyde With sodium dithionite In methanol; water Time; | 99% |
| Stage #1: 1,4-dihydroxy-9,10-anthracenedione With sodium dithionite; potassium hydroxide In methanol; water for 0.25h; Inert atmosphere; Cooling with ice; Stage #2: 3-bromo-4-methoxybenzylaldehyde In methanol; water at 0℃; for 4h; Inert atmosphere; Stage #3: With dihydrogen peroxide In water for 0.333333h; | 76% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol for 24h; Heating; | 98% |
| With methanol; sodium tetrahydroborate at 0℃; | 98% |
| With sodium tetrahydroborate In methanol at 0℃; for 1h; Inert atmosphere; | 97% |
-
-
78-88-6
2,3-Dichloroprop-1-ene

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
72183-81-4
1,4-Bis-(2-chloro-allyloxy)-anthraquinone

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In acetone for 344h; Heating; | 98% |
| With potassium carbonate In N,N-dimethyl-formamide at 70℃; |
-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
107-15-3
ethylenediamine

-
-
20269-39-0
6-hydroxy-1,2,3,4-tetrahydronaphtho<2,3-f>quinoxaline-7,12-dione

| Conditions | Yield |
|---|---|
| copper dichloride In pyridine at 30℃; for 24h; Product distribution; Mechanism; further copper chloride catalyst, effect of oxygen without catalyst, further amine; | 98% |
| With copper dichloride In pyridine at 30℃; for 6h; Product distribution; other diamines, also in the absence of a copper salts; | 98% |
| copper dichloride In pyridine at 30℃; for 24h; | 98% |
| With oxygen; copper dichloride In pyridine at 30℃; for 6h; | 98% |
| With copper dichloride Ambient temperature; | 33% |
-
-
476-60-8
leucoquinizarin

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
122-80-5
N-acetyl-p-phenylenediamine

| Conditions | Yield |
|---|---|
| Stage #1: N-acetyl-p-phenylenediamine With hydrogenchloride In water at 40 - 50℃; for 0.5h; Stage #2: leucoquinizarin; 1,4-dihydroxy-9,10-anthracenedione In water at 85 - 90℃; Solvent; Temperature; | 97.13% |


| Conditions | Yield |
|---|---|
| With leucoquinizarin; boric acid; sodium hydrogensulfite In ethanol; benzene at 62 - 66℃; for 12h; Temperature; Solvent; | 97% |
| With 1-butyl-3-ethyl-1H-imidazolium tetrafluoroborate at 85 - 125℃; Reagent/catalyst; Temperature; Green chemistry; Large scale; | 95.9% |
| With boric acid; calcium carbonate; tin(ll) chloride at 110 - 130℃; |
-
-
421-83-0
trifluoromethane sulfonyl chloride

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
96701-08-5
1,4-Bis-(trifluoromethanesulfonyloxy)-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; 0,2,4-dichlorophenyl-0-methylisopropylamidothiophosphate In dichloromethane at 5 - 10℃; for 0.5h; | 97% |

| Conditions | Yield |
|---|---|
| With indium; water In tetrahydrofuran; methanol at 30℃; Barbier allylation; | 97% |
-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
106-94-5
propyl bromide

-
-
847901-15-9
1,4-dipropoxy-9,10-anthraquinone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 6h; Heating; | 97% |
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 6h; Williamson Ether Synthesis; Inert atmosphere; | 89% |
-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
38399-72-3
leuco-1,4-dihydroxyanthraquinone

-
-
106-50-3
1,4-phenylenediamine

| Conditions | Yield |
|---|---|
| With boric acid In butan-1-ol for 16h; Reagent/catalyst; Reflux; | 97% |

-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
17648-03-2
9,10-Dihydroxy-2,3-dihydro-anthracene-1,4-dione

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-dihydroxy-9,10-anthracenedione With sodium carbonate In water at 85℃; Inert atmosphere; Stage #2: With sodium dithionite In water Inert atmosphere; | 96.1% |
| With sodium disulfite; potassium carbonate Hydrogenation; | 93% |
| With acetic acid; aluminium; indium(III) chloride In ethanol; water at 90℃; for 11h; | 78% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol; triethylamine; acetonitrile byproducts: NEt3*HNO3; a soln. of quinone in Et3N and EtOH were added dropwise under N2 to a soln. of AgNO3 in MeCN-EtOH in the dark at 25°C, the mixt. was stirred for 2 h; filtd., ppt. was washed with cold EtOH, filtrate evapd. under vac.; elem. anal.; | 96% |
-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
106-96-7
propargyl bromide

-
-
1045-75-6
1,4-bis(prop-2-ynyloxy)anthracene-9,10-dione

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 24h; | 96% |
| With potassium carbonate In acetone for 120h; Schlenk technique; Reflux; | 23% |
-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| Stage #1: 1,4-dihydroxy-9,10-anthracenedione With sodium hydroxide In water at 70 - 75℃; for 0.5h; Stage #2: With thiourea In water at 88℃; for 2h; Reagent/catalyst; | 96% |

| Conditions | Yield |
|---|---|
| With acetic acid; sodium sulfate In ethanol for 4h; Reflux; | 95.3% |
| With sodium dithionite; ethanol |

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; tetrafluoroboric acid; bromobutyric acid In methanol at 50 - 55℃; Temperature; Large scale; | 95.1% |
| With ethanol; sodium carbonate; copper(II) sulfate at 140℃; | |
| With sodium hydroxide; copper(II) sulfate at 140℃; |

| Conditions | Yield |
|---|---|
| copper dichloride In pyridine at 30℃; for 24h; | 95% |
| With oxygen; copper dichloride In pyridine at 30℃; for 6h; | 95% |
-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
109-77-3
malononitrile

-
-
86092-18-4
2-amino-3-cyano-5-hydroxyanthra<1,2-b>furan-6,11-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dimethyl sulfoxide Ambient temperature; | 95% |
-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
74-88-4
methyl iodide

-
-
6119-74-0
1,4-dimethoxyanthraquinone

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 40℃; | 95% |
| With aluminum oxide; potassium fluoride In dimethyl sulfoxide at 35℃; for 10h; | 58.5% |
| With potassium carbonate In acetone Reflux; | 31.5% |
-
-
81-64-1
1,4-dihydroxy-9,10-anthracenedione

-
-
13076-29-4
1,4-dimethoxyanthracene

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium borohydrid In methanol | 95% |
| Multi-step reaction with 3 steps 1: 83 percent / K2CO3 / 1,2-dichloro-benzene / 1 h / Heating 2: 100 percent / NaBH4 / methanol; tetrahydrofuran / 0.5 h / 0 °C 3: 40 percent / HCl / tetrahydrofuran / 2 h / 40 °C View Scheme | |
| Multi-step reaction with 2 steps 1: K2CO3 / acetone 2: 62 percent / Zn; glacial acetic acid / 15 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: potassium hydroxide / tetrahydrofuran / 24 h / Reflux 2: zinc; acetic acid / 96 h / Reflux View Scheme |
1,4-DIHYDROXYANTHRAQUINONE Chemical Properties
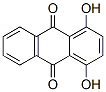
IUPAC Name:1,4-dihydroxyanthracene-9,10-dione
Molecular Formula:C14H8O4
Molecular Weight:240.210920 g/mol
Appearance:red-brown powder
Melting Point:198-199 °C(lit.)
Boiling Point:450 °C
Flash Point:222 °C
vapor density:8.3 (vs air)
vapor pressure:1 mm Hg ( 196.7 °C)
Colour Index:58050
Water Solubility:<1 g/L (20 oC)
Merck:14,8064
BRN:1914036
Synonyms of 1,4-DIHYDROXYANTHRAQUINONE(81-64-1):
1,4-dihydroxy-9,10-anthracenedione;1,4-DIHYDROXYANTHRAQUINONE;1,4 DIHYROXY AQ;DSD ACID;CI NO 58050;CI 58050;LABOTEST-BB LT00052844;PIGMENT VIOLET 12
Categories of 1,4-DIHYDROXYANTHRAQUINONE(81-64-1):
Intermediates of Dyes and Pigments;Anthraquinones, Hydroquinones and Quinones;Anthraquinones;Hydroxyanthraquinones;Solvent Dyestuff
1,4-DIHYDROXYANTHRAQUINONE Uses
1,4-DIHYDROXYANTHRAQUINONE Toxicity Data With Reference
| 1. | eye-rbt 500 mg/24H MLD | 28ZPAK Sbornik Vysledku Toxixologickeho Vysetreni Latek A Pripravku Marhold, J.V.,Institut Pro Vychovu Vedoucicn Pracovniku Chemickeho Prumyclu Praha,Czechoslovakia.: 1972,102. | ||
| 2. | mmo-sat 100 µg/plate | MUREAV Mutation Research. 40 (1976),203. | ||
| 3. | ipr-rat LD50:2100 mg/kg | GTPZAB Gigiena Truda i Professionalnye Zabolevaniia. Labor Hygiene and Occupational Diseases. 21 (12)(1977),27. | ||
| 4. | ivn-mus LD50:320 mg/kg | CSLNX* U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. (Aberdeen Proving Ground, MD 21010) NX#03274 . |
1,4-DIHYDROXYANTHRAQUINONE Consensus Reports
1,4-DIHYDROXYANTHRAQUINONE Safety Profile
Hazard Codes:Xi

Risk Statements:36/37/38-43
36/37/38:Irritating to eyes, respiratory system and skin
43:May cause sensitization by skin contact
Safety Statements:22-24/25-36/37-26
22:Do not breathe dust
24/25:Avoid contact with skin and eyes
36/37:Wear suitable protective clothing and gloves
26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
WGK Germany:2
RTECS:CB6600000
Poison by intravenous route. Moderately toxic by intraperitoneal route. Mutation data reported. An eye irritant. A weak allergen. When heated to decomposition it emits acrid smoke and irritating fumes.
1,4-DIHYDROXYANTHRAQUINONE Specification
First Aid Measures of 1,4-DIHYDROXYANTHRAQUINONE(81-64-1):
Ingestion:If victim is conscious and alert, give 2-4 cupfuls of milk or water. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Skin:Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes.
Eyes:Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Storage of 1,4-DIHYDROXYANTHRAQUINONE(81-64-1):
Keep container closed when not in use. Store in a cool, dry, well-ventilated area away from incompatible substances.
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 816418-32-3
- 816429-58-0
- 816-43-3
- 816444-90-3
- 816454-25-8
- 816456-44-7
- 816458-31-8
- 81646-13-1
- 81647-80-5
- 81-65-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View