-
Name
1,4-Naphthoquinone
- EINECS 204-977-6
- CAS No. 130-15-4
- Article Data386
- CAS DataBase
- Density 1.29 g/cm3
- Solubility Soluble in hot alcohol, ether, benzene, chloroform and carbon disulfide, insoluble in cold water, slightly soluble in petroleum ether
- Melting Point 119-122 °C(lit.)
- Formula C10H6O2
- Boiling Point 297.935 °C at 760 mmHg
- Molecular Weight 158.156
- Flash Point 111.219 °C
- Transport Information UN 2811 6.1/PG 1
- Appearance Yellow needles or brownish green powder with an odor of benzoquinone
- Safety 26-45-61-38-36/37/39-24-16
- Risk Codes 25-26-36/37/38-43-50-34-11
-
Molecular Structure
-
Hazard Symbols
 T+,
T+, N
N
- Synonyms 1,4-Naphthoquinone(8CI);1,4-Dihydro-1,4-diketonaphthalene;NSC 113960;NSC9583;p-Naphthoquinone;a-Naphthoquinone;1,4-Naphthalenedione;
- PSA 34.14000
- LogP 1.62180
Synthetic route

| Conditions | Yield |
|---|---|
| With 2,2'-bipyridylchromium peroxide In benzene for 0.5h; Heating; | 100% |
| With dihydrogen peroxide; bis-(tributyltin oxide) dioxochromium(VI) In benzene at 50℃; for 1.5h; | 100% |
| With 2,2'-bipyridylchromium peroxide In benzene for 0.5h; Product distribution; Heating; effect of various chromium(VI) based oxidants; | 100% |

| Conditions | Yield |
|---|---|
| With oxygen at 340 - 360℃; Product distribution / selectivity; | A 100% B 0.05% |
| With oxygen at 340 - 360℃; | A 100% B 0.1% |
| With oxygen at 340 - 360℃; | A 100% B 0.02% |

| Conditions | Yield |
|---|---|
| With Co(salN-Medpt); oxygen In acetonitrile for 0.5h; | 100% |
| With C126H112O32S6; oxygen In toluene for 1.33333h; Irradiation; | 100% |
| With K10 montmorillonite; iodic acid for 0.333333h; Heating; | 97% |
-
-
10075-62-4
1,4-dimethoxynaphthalene

-
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| With silver(II) oxide; nitric acid In acetone at 20℃; | 100% |
| With N-Bromosuccinimide; sulfuric acid In tetrahydrofuran; water at 20℃; for 0.25h; | 98% |
| With manganese(IV) oxide; nitric acid In dichloromethane for 1.5h; Ambient temperature; | 95% |

| Conditions | Yield |
|---|---|
| With oxone; tetrabutylammomium bromide In water; acetonitrile at 20℃; for 1h; | 100% |
| With silica gel supported cerium(IV) ammonium nitrate-NaBrO3 In dichloromethane; water at 40℃; for 2h; | 95% |
| With Pyridine-2,6-dicarboxylic acid N-oxide; tert.-butylhydroperoxide; ammonium cerium(IV) nitrate In water; acetonitrile at 50℃; for 11h; | 91% |
-
-
106-51-4
p-benzoquinone

-
-
270589-03-2
(E,E)-2-pyridyldimethyl(buta-1,3-dienyl)silane

-
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 20℃; for 38h; | 100% |
| With hydrogenchloride In water at 20℃; for 38h; Product distribution; Further Variations:; Reagents; Solvents; Diels-Alder reaction; | 100% |

| Conditions | Yield |
|---|---|
| With oxygen at 340 - 360℃; | A 100% B 0.01% |
| With oxygen at 340 - 360℃; | A 100% B 0.01% |
| With oxygen at 340 - 360℃; | A 100% B 0.01% |

| Conditions | Yield |
|---|---|
| With oxygen at 340 - 360℃; Product distribution / selectivity; | A 0.01% B 100% C 0.02% |
| With oxygen at 340 - 360℃; | A 0.03% B 100% C 0.05% |
| With oxygen at 340 - 360℃; | A 0.01% B 100% C 0.01% |


| Conditions | Yield |
|---|---|
| With oxygen at 354 - 357℃; | A 0.2% B 100% C 0.15% |


| Conditions | Yield |
|---|---|
| With sodium periodate In water; ethyl acetate at 20℃; for 0.25h; | 98% |
| With [bis(acetoxy)iodo]benzene In acetone at 20℃; for 0.166667h; | 90% |

| Conditions | Yield |
|---|---|
| With H7PMo8V4O40 at 70℃; for 1h; | 96% |
-
-
15448-58-5
2,3-epoxy-1,2β,3β,4-tetrahydronaphthalene-1,4-dione

-
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| With molybdenum hexacarbonyl In toluene for 7.5h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; Dowex 1X8-200 - chloride form In 1,2-dimethoxyethane for 1.41667h; Oxidation; | 93% |
| With Montmorillonite K10; iodic acid at 61℃; for 0.00555556h; microwave irradiation; | 88% |
| With potassium nitrate; trifluoroacetic acid at -20℃; for 0.583333h; | 75% |
| With chromium(III) oxide; sulfuric acid |

| Conditions | Yield |
|---|---|
| With water; potassium bromide In chloroform at 25℃; pH=9; Electrochemical reaction; | 93% |

| Conditions | Yield |
|---|---|
| With water; potassium bromide In chloroform at 25℃; pH=9; Electrochemical reaction; | 92% |
| With N-Bromosuccinimide In water; N,N-dimethyl-formamide at 20 - 80℃; for 16h; | 89% |
| With cerium(IV) ammonium sulphate; sulfuric acid In acetonitrile at 25℃; for 2h; | 42% |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In water; N,N-dimethyl-formamide at 20 - 80℃; for 16h; | 91% |
| With water; potassium bromide In chloroform at 25℃; pH=9; Electrochemical reaction; | 87% |
| With cerium(IV) ammonium sulphate; sulfuric acid In acetonitrile at 25℃; for 24h; | 50% |
| With dihydrogen peroxide; acetic acid auf dem Dampfbad; |

| Conditions | Yield |
|---|---|
| With ceric(IV) tetra-n-butylammonium nitrate; water In dichloromethane at 20℃; for 0.333333h; Reagent/catalyst; Solvent; Temperature; | A 91% B n/a |

| Conditions | Yield |
|---|---|
| With trifluorormethanesulfonic acid; [MnIV(N,N'-di-tert-butyl-2,11-diaza[3.3](2,6)-pyridinophane)(OH)2]2+; tert-butylammonium hexafluorophosphate(V) In 2,2,2-trifluoroethanol; acetone at -30℃; for 0.5h; Kinetics; Temperature; Solvent; Electrochemical reaction; | 90% |
| With ceric methanesulfonate In water; 1,2-dichloro-ethane at 60℃; for 0.583333h; | 89% |
| With perchloric acid; cerium(IV) perchlorate In tetrachloromethane; water; acetonitrile for 1.5h; Oxidation; | 85% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; Dowex 1X8-200 - chloride form In 1,2-dimethoxyethane for 0.75h; Oxidation; | 90% |
| With Montmorillonite K10; iodic acid at 58℃; for 0.00555556h; microwave irradiation; | 72% |
| With bis-[(trifluoroacetoxy)iodo]benzene In water; acetonitrile at 0℃; | 57% |
-
-
1694-92-4
2-Nitrobenzenesulfonyl chloride

-
-
529-34-0
3,4-dihydronaphthalene-1(2H)-one

-
A
-
30904-41-7
o-Nitrobenzenesulfonate

-
B
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| With potassium superoxide In acetonitrile at -35℃; for 6h; | A n/a B 85% |


| Conditions | Yield |
|---|---|
| With potassium nitrososulfonate In chloroform for 9h; pH 6; | 84% |

| Conditions | Yield |
|---|---|
| With silver(II) oxide; nitric acid In acetone at 20℃; | A 83% B 9% |
-
-
3090-45-7
5,8-dihydro-1,4-naphthalenediol

-
A
-
6295-28-9
5,8-dihydronaphthalene-1,4-dione

-
B
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| With H7PMo8V4O40 In chloroform at 70℃; for 1h; Product distribution; Further Variations:; Temperatures; Solvents; reaction times; | A 6% B 82% |

| Conditions | Yield |
|---|---|
| With [bis(acetoxy)iodo]benzene; silica gel; triethylamine at 20℃; for 4h; | 82% |

| Conditions | Yield |
|---|---|
| With potassium peroxymonosulfate; sodium ortho-iodobenzenesulfonate; sodium sulfate; tetra(n-butyl)ammonium hydrogensulfate; potassium carbonate In ethyl acetate at 20℃; for 24h; regioselective reaction; | A 78% B 6% |
| With 2,2,6,6-tetramethylpiperidin-1-oxoammonium chloride In dichloromethane at -80℃; | A 15% B 20% |
| With 3,3-dimethyldioxirane In acetone | A 14% B 17% |
-
-
63383-46-0
1-(trimethylsiloxy)-1,3-butadiene

-
-
5794-62-7
1,4-benzoquinone-2-carboxylic acid

-
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| In dichloromethane for 16h; Ambient temperature; | 78% |

| Conditions | Yield |
|---|---|
| With oxone In water; acetonitrile at 20℃; for 16h; | A 78% B 12% |

-
B
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| With ceric(IV) tetra-n-butylammonium nitrate; water In dichloromethane at 20℃; for 0.333333h; Reagent/catalyst; Solvent; Temperature; | A 78% B n/a |
-
-
571-60-8
1,4-Dihydroxynaphthalene

-
-
57857-70-2
di-(p-methoxyphenyl)tellurium oxide

-
A
-
4456-34-2
bis(4-methoxyphenyl)telluride

-
B
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| In chloroform | A n/a B 76% |

-
-
536-90-3
m-Anisidine

-
-
130-15-4
[1,4]naphthoquinone

-
-
64505-63-1
2-(3-methoxyphenylamino)naphthalene-1,4-dione

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; Reagent/catalyst; | 100% |
| With copper(II) acetate monohydrate; acetic acid at 60 - 70℃; for 0.5h; Under air; | 93% |
| With potassium tert-butylate In N,N-dimethyl-formamide at 20℃; for 2h; | 76% |

| Conditions | Yield |
|---|---|
| With acetic acid; zinc Ambient temperature; sonication, less than 5 min; | 100% |
| With hydrogen; palladium 10% on activated carbon under 2585.81 Torr; for 6h; | 100% |
| With sodium dithionite In diethyl ether; water at 20℃; for 1h; | 100% |
-
-
82167-48-4
2,3-bis<(trimethyksilyl)methyl>-1,3-butadiene

-
-
130-15-4
[1,4]naphthoquinone

-
-
82167-50-8
2,3-Bis-trimethylsilanylmethyl-1,4,4a,9a-tetrahydro-anthraquinone

| Conditions | Yield |
|---|---|
| With hydroquinone In toluene Heating; | 100% |

| Conditions | Yield |
|---|---|
| With chlorine; mercury(II) oxide In tetrachloromethane for 0.5h; | 100% |
| With N-chloro-succinimide; copper(II) chloride monohydrate In acetonitrile at 82℃; for 10.5h; regioselective reaction; | 99.6% |
| With iodine; mercury dichloride; copper dichloride In acetic acid at 60℃; for 3h; | 98% |
-
-
130-15-4
[1,4]naphthoquinone

-
-
20490-14-6
2,3-benzo-1-allyl-1-hydroxycyclohexa-2,5-dien-4-one

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at -23℃; for 3h; | 100% |
-
-
207294-54-0
ethyl 1-acetamido-3-cyclopentene-3-vinyl-1-carboxylate

-
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| With hydroquinone In toluene for 48h; Diels-Alder reaction; Heating; | 100% |
-
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| In benzene Diels-Alder reaction; Heating; | 100% |

| Conditions | Yield |
|---|---|
| With (2S)-2-{diphenyl[(trimethylsilyl)oxy]methyl}pyrrolidine In ethanol; water at -24℃; | 100% |

-
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| With 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate In hexane; dichloromethane at 20℃; for 5h; Reactivity; Reagent/catalyst; Solvent; Darkness; | 100% |

| Conditions | Yield |
|---|---|
| In toluene at 120℃; for 1h; | 100% |
-
-
22483-09-6
2,2-dimethoxyethylamine

-
-
130-15-4
[1,4]naphthoquinone

-
-
1038964-30-5
2-(2,2-dimethoxyethylamino)-1,4-naphthoquinone

| Conditions | Yield |
|---|---|
| With cerium(III) chloride heptahydrate In acetonitrile at 20℃; for 24h; Sonication; | 100% |
-
-
1165952-91-9
cyclohexa-1,3-diene

-
-
130-15-4
[1,4]naphthoquinone

-
-
132016-30-9
endo-tetracyclo[10.2.2.02,11.04.9]hexadeca-4,6,8,13-tetraene-3,10-dione

| Conditions | Yield |
|---|---|
| With methyltrioxorhenium(VII) In acetone for 40h; Ambient temperature; | 99% |
| With scandium tris(trifluoromethanesulfonate); 1-n-butyl-3-methylimidazolium hexafluoroantimonate In dichloromethane at 20℃; for 0.5h; Diels-Alder reaction; | 96% |
| With [O=P(2-py)3W(CO)(NO)2](BF4)2; 3-butyl-1-methyl-1H-imidazol-3-ium hexafluorophosphate at 20℃; for 8h; Diels-Alder reaction; | 93% |
-
-
130-15-4
[1,4]naphthoquinone

-
-
78-79-5
isoprene

-
-
3319-24-2, 55511-73-4
2-methyl-1,4,4a,9a-tetrahydroanthracene-9,10-dione

| Conditions | Yield |
|---|---|
| With methyltrioxorhenium(VII) In acetone for 4h; Ambient temperature; | 99% |
| In methanol at 120℃; Diels-Alder Cycloaddition; Inert atmosphere; Schlenk technique; | 95% |
| In 1-methyl-pyrrolidin-2-one at 190℃; under 750.075 Torr; for 0.0833333h; Diels-Alder reaction; Microwave irradiation; Inert atmosphere; Continuous-flow; | 52% |
-
-
542-92-7
cyclopenta-1,3-diene

-
-
130-15-4
[1,4]naphthoquinone

-
-
24402-95-7
1,4,4a,9a-tetrahydro-1,4-methanoanthracene-9,10-dione

| Conditions | Yield |
|---|---|
| With benzylidene phenylamine; ytterbium(III) triflate In dichloromethane at 0℃; for 24h; Diels-Alder reaction; | 99% |
| With tert-butylammonium hexafluorophosphate(V); calcium(II) trifluoromethanesulfonate In dichloromethane at -20℃; for 4h; Catalytic behavior; Reagent/catalyst; Solvent; Temperature; Time; Diels-Alder Cycloaddition; | 96% |
| In propan-1-ol at 25℃; Thermodynamic data; Rate constant; other solvents and their mixture with water; isobaric activation parameters; |
-
-
109-85-3
2-methoxyethylamine

-
-
130-15-4
[1,4]naphthoquinone

-
-
155859-95-3
2-<(2-methoxyethyl)amino>-1,4-naphthoquinone

| Conditions | Yield |
|---|---|
| In ethanol for 65h; Ambient temperature; | 99% |
| With triethylamine In tetrahydrofuran | 75% |
-
-
513-81-5
2,3-dimethyl-buta-1,3-diene

-
-
130-15-4
[1,4]naphthoquinone

-
-
2670-23-7
2,3-dimethyl-1,4,4a,9a-tetrahydro-9,10-anthracenedione

| Conditions | Yield |
|---|---|
| With methyltrioxorhenium(VII) In acetone for 1h; Ambient temperature; | 99% |
| With tin-tungsten mixed oxide, Sn/W molar ratio = 2, calcined at 800 °C In dichloromethane at 20℃; for 1h; Diels-Alder reaction; Inert atmosphere; | 97% |
| With tetradecafluorohexane In water at 50℃; for 6h; Diels-Alder Cycloaddition; | 93% |

| Conditions | Yield |
|---|---|
| With methyltrioxorhenium(VII) In acetone for 1h; Ambient temperature; | 99% |
-
-
207294-54-0
ethyl 1-acetamido-3-cyclopentene-3-vinyl-1-carboxylate

-
-
130-15-4
[1,4]naphthoquinone

-
-
207294-73-3
2-Acetylamino-6,11-dioxo-2,3,5,5a,6,11,11a,11b-octahydro-1H-cyclopenta[a]anthracene-2-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In benzene for 24h; Heating; | 99% |
-
-
209258-60-6
ethyl 1-acetamido-3,4-dimethylenecyclohepta-1-carboxylate

-
-
130-15-4
[1,4]naphthoquinone

-
-
365224-00-6
9-acetylamino-5,13-dioxo-5a,6,7,8,9,10,11,12,12a,13-decahydro-5H-cyclohepta[b]anthracene-9-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| In benzene for 120h; Diels-Alder reaction; Heating; | 99% |
-
-
139278-54-9
2-(triisopropylsiloxy)-1,3-butadiene

-
-
130-15-4
[1,4]naphthoquinone

| Conditions | Yield |
|---|---|
| (S)-oxazaborolidine-aluminum bromide at -78℃; for 16h; Diels-Alder reaction; | 99% |
| With oxazaborolidinium(1+)*Tf2N(1-) In dichloromethane at -78℃; for 2h; Diels-Alder reaction; | 98% |
1,4-Naphthoquinone Consensus Reports
1,4-Naphthoquinone Specification
1,4-Naphthoquinone is an organic compound with the formula C10H6O2, and its systematic name is the same with the product name. With the CAS registry number 130-15-4, it is also named as p-Naphthoquinone. Its EINECS number is 204-977-6. In addition, the molecular weight is 158.15. Its classification codes are: (1)Agricultural Chemical; (2)Drug / Therapeutic Agent; (3)Fungicide, bactericide, wood preservative; (4)Mutation data; (5)Reproductive Effect; (6)Tumor data. This chemical is used for the production of anthraquinone, and it is a kind of important agricultural fungicide.
Physical properties of 1,4-Naphthoquinone are: (1)ACD/LogP: 1.573; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.57; (4)ACD/LogD (pH 7.4): 1.57; (5)ACD/BCF (pH 5.5): 9.23; (6)ACD/BCF (pH 7.4): 9.23; (7)ACD/KOC (pH 5.5): 170.80; (8)ACD/KOC (pH 7.4): 170.80; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 34.14 Å2; (13)Index of Refraction: 1.617; (14)Molar Refractivity: 42.904 cm3; (15)Molar Volume: 122.551 cm3; (16)Polarizability: 17.008×10-24cm3; (17)Surface Tension: 52.92 dyne/cm; (18)Density: 1.29 g/cm3; (19)Flash Point: 111.219 °C; (20)Enthalpy of Vaporization: 53.782 kJ/mol; (21)Boiling Point: 297.935 °C at 760 mmHg; (22)Vapour Pressure: 0.001 mmHg at 25°C.
Preparation of 1,4-Naphthoquinone: this chemical can be prepared by 4-Amino-[1]naphthol. This reaction will need reagents NaOCl, Dowex 1X8-200 - chloride form and solvent 1,2-dimethoxy-ethane with the reaction time of 85 min. The yield is about 93%.
![1,4-Naphthoquinone can be prepared by 4-Amino-[1]naphthol](/UserFilesUpload/Preparation of 1,4-Naphthoquinone.jpeg)
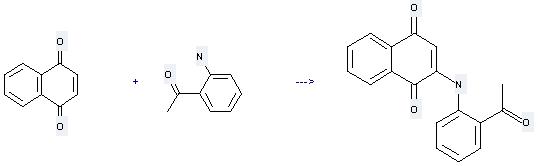
Uses of 1,4-Naphthoquinone: it can be used to produce 2-(2'-acetylanilino)-1,4-naphthoquinone at the ambient temperature. It will need reagents CeCl3·7H2O, air and solvent ethanol with the reaction time of 24 hours. The yield is about 60%.
When you are using this chemical, please be cautious about it as the following:
This chemical is highly flammable, so you should keep it away from sources of ignition - No smoking. It is irritating to eyes, respiratory system and skin, and it may cause sensitisation by skin contact, so you should avoid contact with skin. This substance is toxic if swallowed and is very toxic by inhalation. What's more, it is very toxic to aquatic organisms. It can cause burns. In case of insufficient ventilation, you need to wear suitable respiratory equipment. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. When using it, you need to wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you should seek medical advice immediately (show the label where possible). You must avoid releasing it to the environment, and you need to refer to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C\2c1c(cccc1)C(=O)/C=C/2
(2)Std. InChI: InChI=1S/C10H6O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H
(3)Std. InChIKey: FRASJONUBLZVQX-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| bird - wild | LD50 | oral | 133mg/kg (133mg/kg) | Archives of Environmental Contamination and Toxicology. Vol. 12, Pg. 355, 1983. | |
| guinea pig | LD50 | oral | 400mg/kg (400mg/kg) | Voprosy Kommunal'noi Gigieny. Problems of Communal Hygiene. Vol. 6, Pg. 37, 1966. | |
| guinea pig | LD50 | oral | 400mg/kg (400mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Voprosy Kommunal'noi Gigieny. Problems of Communal Hygiene. Vol. 6, Pg. 37, 1966. |
| mouse | LD50 | intraperitoneal | 5500ug/kg (5.5mg/kg) | Journal of Medicinal Chemistry. Vol. 26, Pg. 570, 1983. | |
| mouse | LDLo | intravenous | 10mg/kg (10mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Journal of Pharmacology and Experimental Therapeutics. Vol. 71, Pg. 210, 1941. |
| mouse | LDLo | oral | 80mg/kg (80mg/kg) | LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Journal of Pharmacology and Experimental Therapeutics. Vol. 71, Pg. 210, 1941. |
| mouse | LDLo | subcutaneous | 40mg/kg (40mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Journal of Pharmacology and Experimental Therapeutics. Vol. 71, Pg. 210, 1941. |
| rat | LD50 | oral | 190mg/kg (190mg/kg) | Voprosy Kommunal'noi Gigieny. Problems of Communal Hygiene. Vol. 6, Pg. 37, 1966. | |
| rat | LD50 | oral | 190mg/kg (190mg/kg) | BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Voprosy Kommunal'noi Gigieny. Problems of Communal Hygiene. Vol. 6, Pg. 37, 1966. |
| rat | LD50 | subcutaneous | 202mg/kg (202mg/kg) | Gigiena Truda i Professional'nye Zabolevaniya. Labor Hygiene and Occupational Diseases. Vol. 16(5), Pg. 52, 1972. | |
| rat | LDLo | intraperitoneal | 50mg/kg (50mg/kg) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 91, 1982. |
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 130161-46-5
- 130-16-5
- 130167-69-0
- 1301-70-8
- 130171-52-7
- 13017-53-3
- 130-17-6
- 130191-91-2
- 13019-20-0
- 13019-22-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View