-
Name
Hexamethylenediamine
- EINECS 204-679-6
- CAS No. 124-09-4
- Article Data185
- CAS DataBase
- Density 0.89 g/cm3
- Solubility Slightly soluble in water, but soluble in ethanol, ethyl ether and benzene
- Melting Point 42-45 °C
- Formula C6H16N2
- Boiling Point 205 °C at 760 mmHg
- Molecular Weight 116.206
- Flash Point 81.1 °C
- Transport Information UN 2735 8/PG 3
- Appearance White to cream solid
- Safety 26-36/37/39-45-22-27
- Risk Codes 34-37-20/21/22
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 1,6-Diamino-n-hexane;1,6-Diaminohexane;Advancure;HMDA;Hexamethylenediamine;Hexylenediamine;Hi Perm;NSC 9257;RT Advancure HD;V 1;a,w-Hexanediamine;
- PSA 52.04000
- LogP 1.86480
Synthetic route

| Conditions | Yield |
|---|---|
| In N-butylamine | 100% |


| Conditions | Yield |
|---|---|
| With hydrogen; Ni(NO3)2, Cu(NO3)2, Cr(NO3)3, Na2CO3; calcined at 380 deg C for 18 h; pretreatment is given in full text In 1,2,4-Trimethylbenzene; ammonia at 80℃; for 24h; Product distribution / selectivity; | A 1.1% B 97% |
| With hydrogen; Ni(NO3)2, Cu(NO3)2, Cr(NO3)3, Na2CO3; calcined at 380 deg C for 18 h In 1,2,4-Trimethylbenzene; ammonia at 80℃; for 24h; Product distribution / selectivity; | A 1.3% B 90.1% |
| With ammonium hydroxide; hydrogen In water; isopropyl alcohol at 130℃; under 41254.1 Torr; for 4h; Autoclave; | A 9% B 90% |
| With hydrogen; iron catalyst at 140℃; under 233483 Torr; |
-
-
1080571-11-4
C6H16N2*C7H16N2O2

-
-
124-09-4
1,6-Hexanediamine

| Conditions | Yield |
|---|---|
| With 2,5-dimethyl-2-ethyl hexanoic acid In water at 120 - 180℃; for 2h; Product distribution / selectivity; | 95% |
-
-
58491-62-6
furan-2,5-dicarbonitrile

-
-
124-09-4
1,6-Hexanediamine

| Conditions | Yield |
|---|---|
| With hydrogen; titanium(IV) oxide; platinum In ethanol at 100℃; under 7500.75 Torr; for 4h; | 95% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; nickel; sodium hydroxide In methanol; water at 30 - 60℃; | 90% |
| With hydrogen In ethanol at 75℃; under 1500.15 - 15001.5 Torr; for 3h; | 80% |
| With ammonia; hydrogen In toluene at 120℃; under 22502.3 Torr; for 16h; Autoclave; | 75% |
-
-
13028-54-1
1,6-diazidohexane

-
-
124-09-4
1,6-Hexanediamine

| Conditions | Yield |
|---|---|
| With aluminum oxide; potassium hydroxide; hydrazine In neat (no solvent) for 1h; Milling; | 90% |

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In methanol at -5 - 0℃; under 41254.1 Torr; for 0.5h; Pressure; Temperature; Autoclave; | 82% |
| With Co-doped zirconium dioxide; ammonia; hydrogen; N-butylamine In methanol at 119.84℃; for 10h; Autoclave; chemoselective reaction; | 63% |

| Conditions | Yield |
|---|---|
| Stage #1: 1,6-hexanediol With chlorocarbonylhydrido[4,5-bis(dicyclohexylphosphinomethyl)acridine]ruthenium(II); ammonia In toluene at 0℃; under 3750.38 Torr; for 1h; Autoclave; Stage #2: In toluene at 155℃; for 16h; Autoclave; | 81% |
| With ammonium hydroxide; 4.04 wt.% ruthenium and 0.7 wt.% rhenium on carbon; ammonia at 160℃; under 5175.52 - 35253.5 Torr; for 3h; Reagent/catalyst; Inert atmosphere; | 23.4% |
| With ammonia at 220 - 260℃; |

| Conditions | Yield |
|---|---|
| With Rh/Al2O3; hydrogen In ethanol at 80℃; for 6h; | A 24.4% B 75.6% |
| With hydrogen; active elemental iron component In ammonia at 70 - 220℃; under 75007.5 - 300030 Torr; | |
| Stage #1: hexanedinitrile With potassium hydroxide; hydrogen; nickel at 50℃; under 15001.5 Torr; Stage #2: With phosphoric acid Purification / work up; |

-
A
-
124-09-4
1,6-Hexanediamine

-
B
-
117839-37-9
6-methoxy-2-(p-tolyl)quinoline

-
C
-
117839-39-1
6-methoxy-4-methyl-2-(p-tolyl)quinoline

| Conditions | Yield |
|---|---|
| In isopropyl alcohol Irradiation; 350 nm; | A 32% B n/a C n/a |

-
-
629-11-8
1,6-hexanediol

-
A
-
111-49-9
hexamethylene imine

-
B
-
124-09-4
1,6-Hexanediamine

-
C
-
4048-33-3
6-amino-1-hexanol

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen In water at 180℃; under 7500.75 - 27752.8 Torr; for 4h; Reagent/catalyst; Solvent; Pressure; Time; | A 19.5% B 23.4% C 17% |
| With ammonia; chlorocarbonylhydrido[4,5-bis(dicyclohexylphosphinomethyl)acridine]ruthenium(II) In toluene at 155℃; under 25502.6 Torr; for 12h; Autoclave; Inert atmosphere; | |
| With ammonia; hydrogen In tert-butyl alcohol at 220℃; under 120012 Torr; for 6h; Kinetics; Reagent/catalyst; | A 66.5 %Chromat. B 21.2 %Chromat. C 6.5 %Chromat. |
| With ammonia; hydrogen In tert-butyl alcohol at 220℃; under 120012 Torr; for 6h; Kinetics; Reagent/catalyst; | A 7.5 %Chromat. B 17.6 %Chromat. C 68.8 %Chromat. |
| With ammonia; hydrogen In tert-butyl alcohol at 220℃; under 120012 Torr; for 6h; Kinetics; | A 23 %Chromat. B 31.3 %Chromat. C 19.1 %Chromat. |

-
-
111-69-3
hexanedinitrile

-
A
-
124-09-4
1,6-Hexanediamine

-
B
-
2432-74-8
1-amino-5-cyanopentane

-
C
-
2321-76-8
2-iminocyclopentanecarbonitrile

| Conditions | Yield |
|---|---|
| With potassium hydroxide; hydrogen; nickel at 50℃; under 15001.5 Torr; Purification / work up; | A n/a B n/a C 12% |


| Conditions | Yield |
|---|---|
| With 1,4-dioxane; barium copper chromite at 260℃; under 147102 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With nickel kieselguhr; ammonia at 200℃; under 147102 Torr; Hydrogenation; | |
| Multi-step reaction with 4 steps 1: sodium hydroxide; water / 3 h / 130 °C 2: tetrahydrofuran / 10 h / 70 °C 3: phosphoric acid; ammonia / 6.5 h / 140 - 280 °C 4: potassium hydroxide; hydrogen; palladium on activated charcoal / ethanol / 75 - 80 °C / 11251.1 Torr View Scheme | |
| Multi-step reaction with 4 steps 1: sodium hydroxide; water / 3 h / 130 °C 2: 2 h / 20 °C 3: phosphorus pentoxide; ammonia / 9.5 h / 140 - 280 °C 4: potassium hydroxide; hydrogen; palladium on activated charcoal / ethanol / 75 - 90 °C / 11251.1 Torr View Scheme | |
| Multi-step reaction with 5 steps 1: sodium hydroxide; water / 3 h / 130 °C 2: 2 h / 47 °C 3: phosphoric acid; ammonia / 5.5 h / 140 - 270 °C 4: potassium hydroxide; hydrogen / ethanol / 75 - 85 °C / 11251.1 Torr 5: ethanol; sodium hydroxide / 2 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With ammonia; hydrogen; nickel at 200℃; unter Druck; | |
| With Isopropylbenzene; ammonia In water |

| Conditions | Yield |
|---|---|
| With silicic acid pumice stone; ammonia; cobalt at 190℃; Hydrogenation; | |
| With silicic acid pumice stone; ammonia; copper at 190℃; Hydrogenation; | |
| With silicic acid pumice stone; ammonia; nickel at 190℃; Hydrogenation; |
-
-
84211-42-7
2,7-diaminooctanedioic acid

-
-
124-09-4
1,6-Hexanediamine

| Conditions | Yield |
|---|---|
| bei der trocknen Destillation; | |
| bei der trocknen Destillation; |
-
-
642470-70-0
N-(5-cyano-pentyl)-benzamide

-
-
124-09-4
1,6-Hexanediamine

| Conditions | Yield |
|---|---|
| durch Reduktion und Verseifung; |
-
-
10513-98-1
1,6-bis-(N-phthalimido)hexane

-
-
124-09-4
1,6-Hexanediamine

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 180 - 190℃; |

| Conditions | Yield |
|---|---|
| With methanol; ozone at -80℃; anschl. Hydrieren des mit NH3 versetzten Reaktionsgemisches an einem Kobalt-Katalysator bei 50grad/200 at; | |
| With ozone; isopropyl alcohol at -80℃; anschl. Hydrieren des mit NH3 versetzten Reaktionsgemisches an einem Kobalt-Katalysator bei 50grad/200 at; | |
| Multi-step reaction with 3 steps 1: dihydrogen peroxide; phosphoric acid; sodium tungstate; hydrogenchloride; 1-hexadecyl-3-methyl-3H-imidazol-1-ium; bromide / water / 4 h / 70 °C 2: sodium periodate; silica gel / dichloromethane / 2 h / 40 °C / Reflux 3: ammonia; hydrogen / methanol / 0.5 h / -5 - 0 °C / 41254.1 Torr / Autoclave View Scheme |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; dimethylborane; ammonia 2.) 0 deg C, 10 min; Yield given. Multistep reaction; |
-
-
111-69-3
hexanedinitrile

-
A
-
111-49-9
hexamethylene imine

-
B
-
124-09-4
1,6-Hexanediamine

-
C
-
2432-74-8
1-amino-5-cyanopentane

| Conditions | Yield |
|---|---|
| With hydrogen; aluminum oxide; nickel at 169.9℃; under 760 Torr; Product distribution; varying space velocity of catalysts; | |
| With hydrogen; nickel monophosphide; silica gel In ethanol at 199.85℃; under 760 Torr; Product distribution; Further Variations:; Catalysts; | A 16 % Chromat. B 65 % Chromat. C 19 % Chromat. |
| With potassium hydroxide; hydrogen; [Ni0.60Mg0.15Al0.25(OH)2](CO32-)0.09(NO3-)0.18 at 79.85℃; under 18751.5 Torr; Product distribution; Further Variations:; Catalysts; Reagents; Temperatures; |

-
-
89333-68-6
6-bis(trimethylsilyl)amino-1-hexene

-
-
124-09-4
1,6-Hexanediamine

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; dimethylborane; ammonia 1.) 0 deg C, 1 h, 2.) O deg C; Yield given. Multistep reaction; |
-
-
121511-53-3
N,N'-Bis-[1-(4-hexadecyloxy-phenyl)-meth-(E)-ylidene]-hexane-1,6-diamine

-
A
-
124-09-4
1,6-Hexanediamine

-
B
-
59117-18-9
p-n-hexadecyloxybenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water; cetyltrimethylammonim bromide In chloroform at 25℃; Rate constant; | |
| With 2-methyl-propan-1-ol; sulfuric acid; sodium dodecyl-sulfate In hexane; water at 27℃; Kinetics; |
-
-
121511-46-4
N,N'-Bis-[1-(2-hexadecyloxy-phenyl)-meth-(E)-ylidene]-hexane-1,6-diamine

-
A
-
124-09-4
1,6-Hexanediamine

-
B
-
5376-76-1
o-(hexadecyloxy)benzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water; cetyltrimethylammonim bromide In chloroform at 25℃; Rate constant; | |
| With 2-methyl-propan-1-ol; sulfuric acid; sodium dodecyl-sulfate In hexane; water at 27℃; Kinetics; |

| Conditions | Yield |
|---|---|
| With buffer In water at 25℃; Rate constant; Mechanism; SIE; |

-
-
124-09-4
1,6-Hexanediamine

| Conditions | Yield |
|---|---|
| With ethanol; sodium anschl. mit Benzoylchlorid und Erhitzen mit HCl auf 170-180grad; | |
| With ethanol; sodium anschl. mit Benzoylchlorid und Erhitzen mit HCl auf 170-180grad; |

-
-
124-09-4
1,6-Hexanediamine

| Conditions | Yield |
|---|---|
| With tetrahydrofuran; nickel Fuller's earth-catalyst; ammonia at 110 - 120℃; under 36775.4 Torr; Hydrogenation; | |
| With methanol; ammonia; cobalt at 60 - 80℃; under 110326 Torr; Hydrogenation; |


| Conditions | Yield |
|---|---|
| 100% | |
| MnZSM-5(30) zeolite In water at 400℃; Cyclization; | 95.6% |
| With tris(triphenylphosphine)ruthenium(II) chloride In diphenylether at 180℃; for 5h; | 78% |

| Conditions | Yield |
|---|---|
| In methanol | 100% |
| In toluene for 3h; Heating; | 93% |
-
-
124-09-4
1,6-Hexanediamine

-
-
150785-44-7
N,N'-bis(tert-butoxycarbonyl)-N-(γ,γ-dimethylallyl)-S-methylisothiourea

-
-
623580-13-2
N-(γ,γ-dimethylallyl)-N,N'-bis-(tert-butoxycarbonyl)-N''-(6-aminohexyl)-guanidine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 50℃; for 3h; | 100% |
| In tetrahydrofuran; water at 50℃; for 1h; | 72% |
| In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; benzotriazol-1-ol at 20℃; for 1h; Acylation; | 100% |
-
-
124-09-4
1,6-Hexanediamine

-
-
1571-08-0
methyl 4-formylbenzoate

-
-
106872-64-4
1,6-bis(4-methoxycarbonylbenzylidene)hexanediamine

| Conditions | Yield |
|---|---|
| In toluene for 0.5h; Condensation; Heating; | 100% |
| In toluene for 1h; Reflux; Dean-Stark trap; | 100% |
-
-
124-09-4
1,6-Hexanediamine


| Conditions | Yield |
|---|---|
| In ethanol; water at 70℃; for 24h; | 100% |
-
-
124-09-4
1,6-Hexanediamine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
16644-54-5
N,N'-bis(tert-butoxycarbonyl)-1,6-hexanediamine

| Conditions | Yield |
|---|---|
| Stage #1: 1,6-Hexanediamine With triethylamine at 20℃; for 0.5h; Inert atmosphere; Stage #2: di-tert-butyl dicarbonate at 20℃; for 12h; Inert atmosphere; | 100% |
| In methanol at 20℃; for 1h; | 91% |
| With succinimidinium N-sulfonic acid hydrogen sulfate In neat (no solvent) at 20℃; for 0.25h; Green chemistry; | 89% |
| In 1,4-dioxane at 20℃; for 48h; | |
| With hydrogenchloride In methanol at 0 - 20℃; |

| Conditions | Yield |
|---|---|
| With triethylamine In methanol; N,N-dimethyl-formamide at 20℃; for 6h; | 100% |
-
-
124-09-4
1,6-Hexanediamine

-
-
886448-06-2
C12H21NO2

-
-
297748-90-4
N,N'-bis[1-n-propoxy-2,2,6,6-tetramethylpiperidin-4-yl]hexane-1,6-diamine

| Conditions | Yield |
|---|---|
| With hydrogen; platinum on carbon In ethanol at 100℃; under 37503.8 Torr; | 100% |

| Conditions | Yield |
|---|---|
| 100% |
-
-
124-09-4
1,6-Hexanediamine

-
-
1737-93-5
3,5-dichloro-2,4,6-trifluoropyridine

-
-
54981-54-3
N-(3,5-Dichlor-2,6-difluor-4-pyridyl)-hexan-1,6-diamin

| Conditions | Yield |
|---|---|
| In methanol; ethanol excess of alkyldiamine in CH3OH, 50 - 70 °C, 20 min stirring;; | 100% |
| In methanol; ethanol |
-
-
124-09-4
1,6-Hexanediamine

-
-
322474-21-5
N,N'-bis-Boc-S-methyl-isothiourea

-
-
623580-13-2
N-(γ,γ-dimethylallyl)-N,N'-bis-(tert-butoxycarbonyl)-N''-(6-aminohexyl)-guanidine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 50℃; for 3h; | 100% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In ethanol for 24h; | 100% |

| Conditions | Yield |
|---|---|
| In dichloromethane | 100% |
-
-
124-09-4
1,6-Hexanediamine

-
-
598-21-0
2-Bromoacetyl bromide

-
-
6722-88-9
N,N′-(hexane-1,6-diyl) bis(2-bromoethanamide)

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; water at 5 - 20℃; for 12h; | 100% |
| With potassium carbonate In chloroform; water at 5 - 20℃; for 24.5h; | 99% |
| In chloroform; water at 5℃; for 2h; |
-
-
124-09-4
1,6-Hexanediamine

-
-
617-09-4
di-o-tolyl carbonate

-
-
6030-54-2
1,6-bis(methoxycarbonylamino)hexane

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol | 99.9% |
-
-
124-09-4
1,6-Hexanediamine

-
-
2050-95-5
diisoamyl carbonate

-
-
16644-34-1
N,N'-hexanediyl-bis-carbamic acid bis(3-methylbutyl) ester

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol at 80℃; Product distribution / selectivity; Industry scale; | 99.7% |
| With sodium methylate In methanol at 80℃; Product distribution / selectivity; Inert atmosphere; | |
| With sodium methylate In methanol at 80℃; Product distribution / selectivity; Inert atmosphere; | |
| With sodium methylate In methanol at 80℃; Product distribution / selectivity; Inert atmosphere; | |
| With sodium methylate In methanol at 80℃; Product distribution / selectivity; | 99.7 %Chromat. |

| Conditions | Yield |
|---|---|
| With phosgene In chlorobenzene at 10 - 130℃; for 4 - 5h; Solvent; | 99.6% |
-
-
102-09-0
bis(phenyl) carbonate

-
-
124-09-4
1,6-Hexanediamine

-
-
4223-31-8
N,N′-hexanediyl bis-carbamic acid diphenyl ester

| Conditions | Yield |
|---|---|
| With lead(II) oxide In phenol at 50℃; Product distribution / selectivity; Industry scale; | 99.5% |
| With phenol at 50℃; Product distribution / selectivity; Industry scale; | 99.5% |
| With phenol at 50℃; Industrial scale; | 99.5% |
-
-
124-09-4
1,6-Hexanediamine

-
-
616-38-6
carbonic acid dimethyl ester

-
-
6030-54-2
1,6-bis(methoxycarbonylamino)hexane

| Conditions | Yield |
|---|---|
| With hydrogenchloride; sodium methylate In methanol | 99.5% |
| With sodium methylate In methanol at 50℃; for 2.5h; | 98.1% |
| With water; sodium methylate In methanol at 50℃; for 5.5h; | 98.1% |
-
-
124-09-4
1,6-Hexanediamine

-
-
1422-70-4
bis(2,2,3,3-tetrafluoropropyl) carbonate

-
-
384812-59-3
C14H20F8N2O4

| Conditions | Yield |
|---|---|
| at 70℃; for 1h; | 99.4% |
-
-
124-09-4
1,6-Hexanediamine

-
-
50903-59-8
N-t-butyloxycarbonyl-Nε-benzyloxycarbonyl-lysine pentafluorophenyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1,6-Hexanediamine; N-t-butyloxycarbonyl-Nε-benzyloxycarbonyl-lysine pentafluorophenyl ester In N,N-dimethyl-formamide at 20℃; Stage #2: With diethylaminopropylamine In N,N-dimethyl-formamide for 0.5h; | 99.16% |
-
-
124-09-4
1,6-Hexanediamine

-
-
110-13-4
2,5-hexanedione

-
-
6970-82-7
1,6-bis(2,5-dimethyl-1H-pyrrol-1-yl)hexane

| Conditions | Yield |
|---|---|
| In neat (no solvent) at 20℃; for 0.083h; Concentration; Paal-Knorr Pyrrole Synthesis; Green chemistry; | 99% |
| With gallium(III) triflate In neat(no solvent) at 30℃; for 0.583333h; Paal-Knorr condensation; | 94% |
| scandium tris(trifluoromethanesulfonate) at 30℃; Paal-Knorr reaction; | 93% |

| Conditions | Yield |
|---|---|
| With phenol at 50 - 120℃; for 3.5h; Reagent/catalyst; Temperature; Inert atmosphere; | 99% |
| In toluene at 90 - 220℃; under 9750.98 Torr; Industry scale; | 98% |
| With N-benzyl-trimethylammonium hydroxide In 2-methoxy-ethanol at 100℃; for 3h; Reagent/catalyst; Solvent; Temperature; Time; Concentration; | 97.2% |
-
-
124-09-4
1,6-Hexanediamine

-
-
88579-78-6
2,5-di-iodo-1,1,1,6,6,6-hexafluoro-3,4-diazahexa-2,4-diene

| Conditions | Yield |
|---|---|
| In diethyl ether for 26h; Ambient temperature; | 99% |
-
-
102-09-0
bis(phenyl) carbonate

-
-
124-09-4
1,6-Hexanediamine

-
-
108-95-2
phenol

-
-
4223-31-8
N,N′-hexanediyl bis-carbamic acid diphenyl ester

| Conditions | Yield |
|---|---|
| at 45 - 60℃; for 1.33333h; Product distribution / selectivity; | 99% |
| at 50 - 60℃; for 1.33333h; Product distribution / selectivity; |

-
-
124-09-4
1,6-Hexanediamine

-
-
107-13-1
acrylonitrile

-
-
51851-40-2
N,N,N',N'-(tetrakis(cyanoethyl)hexamethylenediamine)

| Conditions | Yield |
|---|---|
| In methanol at 50℃; for 48h; | 99% |
| In water at 20 - 80℃; for 6h; Michael addition; | 96% |
| Stage #1: 1,6-Hexanediamine; acrylonitrile With acetic acid In ethanol for 11h; Heating / reflux; Stage #2: With ammonia In water; ethyl acetate at 20℃; |
-
-
124-09-4
1,6-Hexanediamine

-
-
1609-86-5
Tert-butyl isocyanate

-
-
15772-96-0
tBu-NH-CO-NH-(CH2)6-NH-CO-NH-tBu

| Conditions | Yield |
|---|---|
| In chloroform for 1h; | 99% |
-
-
124-09-4
1,6-Hexanediamine

-
-
58-85-5
biotin

-
-
65953-56-2
N-(6-aminohexyl)-5-((3aS,4S,6aR)-3a,6a-dimethyl-2-oxohexahydro-1H-thieno[3,4-d] imidazol-4-yl)pentanamide

| Conditions | Yield |
|---|---|
| Stage #1: biotin With di(succinimido) carbonate; triethylamine In N,N-dimethyl-formamide at 20℃; for 6h; Inert atmosphere; Stage #2: 1,6-Hexanediamine In N,N-dimethyl-formamide Inert atmosphere; | 99% |
1,6-Hexanediamine Specification
This product is an organic compound with the formula C6H16N2. The systematic name of this chemical is 1,6-Hexanediamine. It belongs to the product categories of Industrial/Fine Chemicals; alpha,omega-Alkanediamines; alpha,omega-Bifunctional Alkanes; Monofunctional & alpha,omega-Bifunctional Alkanes. Its EINECS number is 204-679-6. With the CAS registry number 124-09-4, it is also named as Hexamethylenediamine. In addition, the molecular weight is 116.20. Its classification code is Reproductive Effect. It is stable in air but combustible. Like all organic compounds, it is incompatible with strong oxidants. It should be sealed and stored in a ventilated and dry place. Moreover, it should be protected from heat, moisture and fire. Hexamethylenediamine is used almost exclusively for the production of polymers, an application that takes advantage of its bifunctional structure. The great majority of the diamine is consumed by the production of nylon 6-6 via condensation with adipic acid. Otherwise hexamethylene diisocyanate (HDI) is generated from this diamine as a monomer feedstock in the production of polyurethane. The diamine also serves as a cross-linking agent in epoxy resins.
Physical properties of 1,6-Hexanediamine are: (1)ACD/LogP: 0.04; (2)# of Rule of 5 Violations: 0; (3)ACD/BCF (pH 5.5): 1; (4)ACD/BCF (pH 7.4): 1; (5)ACD/KOC (pH 5.5): 1; (6)ACD/KOC (pH 7.4): 1; (7)#H bond acceptors: 2; (8)#H bond donors: 4; (9)#Freely Rotating Bonds: 7; (10)Polar Surface Area: 52.04 Å2; (11)Index of Refraction: 1.459; (12)Molar Refractivity: 36.91 cm3; (13)Molar Volume: 134.9 cm3; (14)Polarizability: 14.63×10-24cm3; (15)Surface Tension: 35.3 dyne/cm; (16)Density: 0.861 g/cm3; (17)Flash Point: 81.1 °C; (18)Enthalpy of Vaporization: 44.13 kJ/mol; (19)Boiling Point: 205 °C at 760 mmHg; (20)Vapour Pressure: 0.256 mmHg at 25°C.
Preparation of 1,6-Hexanediamine: this chemical can be prepared by hexanedinitrile with hydrogen generated in situ electrochemically on Raney nickel electrod. This reaction will need reagents H2, NaOMe and solvent methanol. This reaction will also need catalyst Raney nickel. The yield is about 70%.
![]()
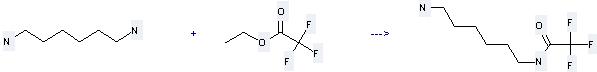
Uses of 1,6-Hexanediamine: it can be used to produce N-(6-aminohexyl)trifluoroacetamide at the temperature of -78 - 0 °C. It will need solvent methanol with the reaction time of 2.5 hours. The yield is about 95%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation, in contact with skin and if swallowed. It is irritating to respiratory system and can cause burns. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. You should not breathe dust and must take off immediately all contaminated clothing. When using it, you need wear suitable protective clothing, gloves and eye/face protection. In case of accident or if you feel unwell, you need seek medical advice immediately (show the label where possible).
You can still convert the following datas into molecular structure:
(1)SMILES: NCCCCCCN
(2)Std. InChI: InChI=1S/C6H16N2/c7-5-3-1-2-4-6-8/h1-8H2
(3)Std. InChIKey: NAQMVNRVTILPCV-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LCLo | inhalation | 750mg/m3/10M (750mg/m3) | National Defense Research Committee, Office of Scientific Research and Development, Progress Report.Vol. NDCrc-132, Pg. SEP1942。 | |
| mouse | LD50 | intraperitoneal | 320mg/kg (320mg/kg) | Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 43(11), Pg. 110, 1978. | |
| mouse | LD50 | intravenous | 180mg/kg (180mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#02313。 | |
| mouse | LD50 | subcutaneous | 1300mg/kg (1300mg/kg) | "Toxicometric Parameters of Industrial Toxic Chemicals Under Single Exposure," Izmerov, N.F., et al., Moscow, Centre of International Projects, GKNT, 1982Vol. -, Pg. 74, 1982. | |
| rabbit | LD50 | skin | 1110mg/kg (1110mg/kg) | Toxicology and Applied Pharmacology. Vol. 42, Pg. 417, 1977. | |
| rat | LC | inhalation | > 950mg/m3/4H (950mg/m3) | United States Environmental Protection Agency, Office of Pesticides and Toxic Substances. Vol. 8EHQ-1090-0979. | |
| rat | LD50 | oral | 750mg/kg (750mg/kg) | Toxicology and Applied Pharmacology. Vol. 42, Pg. 417, 1977. |
Related Products
- 10,10'-Bis([1,1'-biphenyl]-4-yl)-9,9'-bianthracene
- 10,10'-Dibromo-9,9'-bianthryl
- 10,10-Dimethylanthrone
- 10,10-Oxybisphenoxarsine
- 10-[1,1'-Biphenyl]-4-yl-2-(1-methylethyl)-9-oxo-9H-thioxanthenium hexafluorophosphate
- 10,11-Dehydroimipramine
- 10,11-Dihydro-11-oxodibenzo[b,f][1,4]thiazepine
- 10,11-Dihydro-2-(4-methyl-1-piperazinyl)-11-(2-athiazolyl)-pyridazino(3,4-b)(1,4)benzoxazepine
- 10,11-DIHYDRO-2-(4-METHYL-1-PIPERAZINYL)-11-(3,4-XYLYL)PYRIDAZINO(3,4b)(1,4)-BENZOXAZEPINE MALEATE
- 10,11-Dihydro-5-(3-dimethylamino-2-methylpropyl)-5h-dibenz (b,f)azepine
- 12410-14-9
- 124-10-7
- 124111-47-3
- 12411-64-2
- 124-11-8
- 124123-15-5
- 12412-58-7
- 124-12-9
- 124-13-0
- 124148-15-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View