-
Name
3-CHLORO-O-XYLENE
- EINECS 229-843-4
- CAS No. 608-23-1
- Article Data22
- CAS DataBase
- Density 1.049 g/cm3
- Solubility 20.1mg/L at 24℃
- Melting Point -41.95°C (estimate)
- Formula C8H9Cl
- Boiling Point 186 °C at 760 mmHg
- Molecular Weight 140.612
- Flash Point 63.6 °C
- Transport Information
- Appearance Clear colourless to light yellow liquid
- Safety 26-36/37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1,2-Dimethyl-3-chlorobenzene;1-Chloro-2,3-dimethylbenzene;2,3-Dimethylchlorobenzene;3-Chloro-1,2-dimethylbenzene;3-Chloro-o-xylene;o-Xylene,3-chloro- (8CI);
- PSA 0.00000
- LogP 2.95680
Synthetic route

| Conditions | Yield |
|---|---|
| With chlorine; iron(III) chloride; 1,3,7,9-tetrachloro-2,8-dimethylphenoxathiin at -5 - 20℃; for 2.5 - 5h; Product distribution / selectivity; | A 61.2% B 10.8% |
| With chlorine; iron(III) chloride at 22℃; for 2h; Product distribution; study of the effect of the nature of the catalysts (AlCl3, SbCl3, SbCl5, Fe, I2) on the composition of the reaction mass; | |
| With iodine beim Chlorieren; |
-
-
36018-19-6
2,3-dimethylphenyl chloroformate

-
-
608-23-1
3-chloro-o-xylene

| Conditions | Yield |
|---|---|
| With aluminium trichloride In various solvent(s) at 200℃; for 3.5h; | 60% |
-
-
78071-51-9
cis-3,4-dimethylhexa-1,5-diyn-3-ene

-
A
-
608-23-1
3-chloro-o-xylene

-
B
-
52331-02-9
1,4-dichloro-2,3-dimethyl-benzene

| Conditions | Yield |
|---|---|
| With tetrachloromethane at 190℃; | A 5% B 20% |

| Conditions | Yield |
|---|---|
| With disulfur dichloride; aluminium trichloride; sulfuryl dichloride | |
| With sulfuryl dichloride |

| Conditions | Yield |
|---|---|
| With hydrogenchloride Diazotization.Behandlung der Diazoniumsalz-Loesung mit CuCl; |
-
-
95-47-6
o-xylene

-
-
7553-56-2
iodine

-
A
-
615-60-1
4-chloro-1,2-dimethylbenzene

-
B
-
608-23-1
3-chloro-o-xylene

| Conditions | Yield |
|---|---|
| beim Chlorieren; |

-
-
95-47-6
o-xylene

-
-
7782-50-5
chlorine

-
-
64-19-7
acetic acid

-
A
-
615-60-1
4-chloro-1,2-dimethylbenzene

-
B
-
608-23-1
3-chloro-o-xylene

| Conditions | Yield |
|---|---|
| at 25℃; Rate constant; |


| Conditions | Yield |
|---|---|
| With chlorine; tetrachlorinated 2,8-dimethylphenoxathiine at 20℃; for 5h; | A n/a B 71.5 %Chromat. C n/a |

-
-
95-47-6
o-xylene

-
A
-
95-66-9
2,4-dimethylchlorobenzene

-
B
-
556-97-8
1-chloro-3,5-dimethylbenzene

-
C
-
95-72-7
2-chloro-1,4-dimethyl-benzene

-
D
-
6781-98-2
2,6-dimethyl-1-chlorobenzene

-
E
-
615-60-1
4-chloro-1,2-dimethylbenzene

-
F
-
608-23-1
3-chloro-o-xylene

-
G
-
20824-80-0
1,2-dichloro-4,5-dimethyl-benzene

-
H
-
68266-67-1
1,2-dichloro-3,4-dimethyl-benzene

-
I
-
34060-72-5
2-methyl-5-chlorobenzyl chloride

-
J
-
52331-02-9
1,4-dichloro-2,3-dimethyl-benzene

-
K
-
70172-92-8
3,5-dichloro-o-xylene

-
L
-
552-45-4
1-chloromethyl-2-methylbenzene

-
M
-
55676-90-9
alpha,2-dichloro-6-methyltoluene

| Conditions | Yield |
|---|---|
| With chlorine; the fluorine-containing K-L-type zeolite In 1,2-dichloro-ethane at 80℃; for 1.75 - 3.66667h; Product distribution / selectivity; | |
| With chlorine; a K-L-type zeolite In 1,4-dioxane; 1,2-dichloro-ethane at 80℃; for 6.75h; |

-
-
95-47-6
o-xylene

-
A
-
615-60-1
4-chloro-1,2-dimethylbenzene

-
B
-
608-23-1
3-chloro-o-xylene

-
C
-
552-45-4
1-chloromethyl-2-methylbenzene

| Conditions | Yield |
|---|---|
| With N-chloro-succinimide; copper dichloride at 102℃; under 2625.26 Torr; for 0.0666667h; Microwave irradiation; Darkness; chemoselective reaction; |

-
-
64-19-7
acetic acid

-
-
87-59-2
2,3-Dimethylaniline

-
A
-
526-75-0
2,3-Dimethylphenol

-
B
-
608-23-1
3-chloro-o-xylene

-
C
-
22618-22-0
2,3-dimethylphenyl acetate

| Conditions | Yield |
|---|---|
| Stage #1: 2,3-Dimethylaniline With hydrogenchloride; acetic acid; sodium nitrite In water at 0 - 5℃; Stage #2: acetic acid With hydrogenchloride; water; copper(l) chloride; calcium oxide; crotonaldehyde In acetone at 0 - 20℃; for 5h; |

-
-
608-23-1
3-chloro-o-xylene

-
-
22479-40-9
1,2-bis(bromomethyl)-6-chlorobenzene

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In tetrachloromethane Irradiation; | 100% |
| With N-Bromosuccinimide In tetrahydrofuran Irradiation; Reflux; | 89% |
| With bromine |

| Conditions | Yield |
|---|---|
| With potassium carbonate; PdCl2[diisopropyl(o-isopropylphenyl)phosphane]2 In water; N,N-dimethyl-formamide at 150℃; for 0.5h; Suzuki-Miyaura coupling reaction; microwave irradiation; | 98% |
| With C26H29Br2Cl2N5Pd2; caesium carbonate In water; N,N-dimethyl-formamide for 1.5h; Time; Suzuki-Miyaura Coupling; | 69% |
-
-
608-23-1
3-chloro-o-xylene

-
-
73183-34-3
bis(pinacol)diborane

-
-
1232132-73-8
2-(2,3-dimethylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 2-(2-methoxyphenyl)-1-methyl-3-(diphenylphosphino)-1H-indole; cesium acetate In 1,4-dioxane at 110℃; for 24h; Inert atmosphere; | 93% |
-
-
608-23-1
3-chloro-o-xylene

-
-
637-44-5
phenylpropyolic acid

-
-
1262044-52-9
1,2-dimethyl-3-(phenylethynyl)benzene

| Conditions | Yield |
|---|---|
| With [Pd((η5-C5H5)Fe[(η5-C5H3)C(Me)=N(C6H4-4-Me)])(μ-Cl)]2; potassium carbonate; XPhos In 5,5-dimethyl-1,3-cyclohexadiene; water at 120℃; for 3h; | 91% |

| Conditions | Yield |
|---|---|
| With hydrogen; 5%-palladium/activated carbon at 290 - 320℃; for 1h; | 90% |
-
-
608-23-1
3-chloro-o-xylene

-
-
134640-85-0
2,3-dimethylphenylmagnesium bromide

-
-
7495-46-7
2,2′,3,3′-tetramethylbiphenyl

| Conditions | Yield |
|---|---|
| With iron(III)-acetylacetonate In tetrahydrofuran at 80℃; for 0.5h; Flow reactor; Industrial scale; | 90% |
-
-
608-23-1
3-chloro-o-xylene

-
-
105365-51-3
4-butoxyphenylboronic acid

| Conditions | Yield |
|---|---|
| With potassium phosphate; 3-(dicyclohexylphosphino)-2-(2,6-dimethoxyphenyl)-1-methyl-1H-indole; palladium diacetate In 1,4-dioxane at 100℃; for 24h; Suzuki-Miyaura Coupling; Schlenk technique; Sealed tube; Inert atmosphere; | 90% |
-
-
615-60-1
4-chloro-1,2-dimethylbenzene

-
-
608-23-1
3-chloro-o-xylene

-
-
5006-39-3
2,3,3’,4’-tetramethyl-1,1‘-biphenyl

| Conditions | Yield |
|---|---|
| With iodine; magnesium; triphenylphosphine at 110℃; for 7h; Inert atmosphere; | 88% |
-
-
608-23-1
3-chloro-o-xylene

-
-
100379-00-8
2,6-dimethylbenzene boronic acid

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); potassium phosphate monohydrate; 2-(2-methoxyphenyl)-1-methyl-3-(diphenylphosphino)-1H-indole In 1,4-dioxane at 110℃; for 24h; Suzuki-Miyaura coupling; Inert atmosphere; | 87% |

| Conditions | Yield |
|---|---|
| With 4,4'-bipyridine; isopropylmagnesium chloride; magnesium; nickel dichloride at 115℃; for 8h; Inert atmosphere; | 85% |
| With nickel(II) triflate; lithium; magnesium; ethylene dibromide In tetrahydrofuran at 20℃; Reflux; Inert atmosphere; | 70% |
| Stage #1: 3-chloro-o-xylene With iodine; magnesium In tetrahydrofuran at 110℃; for 10h; Inert atmosphere; Stage #2: With iron(III) chloride In tetrahydrofuran at 60℃; for 12h; | 50% |
-
-
608-23-1
3-chloro-o-xylene

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

-
-
5779-93-1
2,3-dimethylbenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-o-xylene With magnesium In tetrahydrofuran for 5h; Inert atmosphere; Reflux; Stage #2: N,N-dimethyl-formamide In tetrahydrofuran at 20 - 30℃; for 5h; | 82% |

| Conditions | Yield |
|---|---|
| With N-methyl-2-phenyl-3-(dicyclohexylphosphino)-1H-indole; palladium diacetate; triethylamine; sodium t-butanolate In water at 100℃; for 18h; Suzuki-Miyaura Coupling; Inert atmosphere; Schlenk technique; | 80% |

| Conditions | Yield |
|---|---|
| With 3-(dicyclohexylphosphino)-2-(2-methoxyphenyl)-1-methyl-1-H-indole; tetrabutyl ammonium fluoride; palladium diacetate at 110℃; for 3h; Hiyama Coupling; Schlenk technique; Inert atmosphere; Sealed tube; | 80% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-o-xylene With ethylmagnesium bromide; magnesium; lithium chloride In tetrahydrofuran at 40 - 50℃; for 10h; Stage #2: acetic anhydride In tetrahydrofuran; toluene at -5 - 5℃; for 2h; Temperature; Inert atmosphere; | 79.2% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-o-xylene With iodine; magnesium; ethylene dibromide In tetrahydrofuran for 2h; Grignard Reaction; Heating / reflux; Stage #2: 1-bromo-4-tert-butylbenzene; nickel dichloride In tetrahydrofuran at 50 - 55℃; for 2h; | 75% |

| Conditions | Yield |
|---|---|
| With potassium phosphate; tris-(dibenzylideneacetone)dipalladium(0); 3-(dicyclohexylphosphino)-2-(2,6-dimethoxyphenyl)-1-methyl-1H-indole In 1,4-dioxane at 100℃; for 2h; Suzuki-Miyaura Coupling; Schlenk technique; Sealed tube; Inert atmosphere; | 70% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene; palladium dichloride; XPhos In dimethyl sulfoxide at 120℃; for 24h; Inert atmosphere; | 68% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-chloro-o-xylene; boron triiodide at 160℃; for 16h; Inert atmosphere; Schlenk technique; Stage #2: 2,3-dimethyl-2,3-butane diol With triethylamine at 0 - 20℃; for 0.5h; regioselective reaction; | 66% |


| Conditions | Yield |
|---|---|
| With oxygen at 100 - 105℃; under 750.075 Torr; for 1h; Temperature; Pressure; Reagent/catalyst; Autoclave; | 60% |

| Conditions | Yield |
|---|---|
| With sodiumsulfide nonahydrate; benzyltrimethylammonium chloride In dichloromethane Reflux; | 55% |
-
-
608-23-1
3-chloro-o-xylene

-
-
73183-34-3
bis(pinacol)diborane

-
A
-
95-47-6
o-xylene

-
B
-
1232132-73-8
2-(2,3-dimethylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With bis(1,3-dimesityl-1H-imidazol-2(3H)-ylidene)nickel(0); potassium methanolate In hexane at 25℃; for 6h; Inert atmosphere; Irradiation; | A 18 %Chromat. B 55% C 7 %Chromat. |

-
-
608-23-1
3-chloro-o-xylene

-
-
27563-65-1
3-chlorobenzene-1,2-dicarboxylic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate; cetyltrimethylammonim bromide In water at 50 - 60℃; for 24h; | 40% |
| With potassium permanganate; water; cetyltrimethylammonim bromide for 120h; Reflux; | 35% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; tri-tert-butyl phosphine; palladium diacetate In N,N-dimethyl-formamide; toluene at 100℃; for 21h; Product distribution / selectivity; | 4% |
-
-
1066-35-9
dimethylmonochlorosilane

-
-
608-23-1
3-chloro-o-xylene

-
-
17961-79-4
(2,3-dimethyl-phenyl)-trimethyl-silane

| Conditions | Yield |
|---|---|
| With sodium |
-
-
608-23-1
3-chloro-o-xylene

-
-
68266-67-1
1,2-dichloro-3,4-dimethyl-benzene

| Conditions | Yield |
|---|---|
| With iron at -10℃; Einleiten von Chlor; |
-
-
608-23-1
3-chloro-o-xylene

-
-
75-36-5
acetyl chloride

-
-
15089-73-3
1-(2-chloro-4,5-dimethyl-phenyl)-ethanone

| Conditions | Yield |
|---|---|
| With aluminium trichloride |
1-Chloro-2,3-dimethylbenzene Specification
The CAS registry number of Benzene,1-chloro-2,3-dimethyl- is 608-23-1. The systematic name is 1-chloro-2,3-dimethylbenzene. In addition, the molecular formula is C8H9Cl and the molecular weight is 140.61. It is also called 3-Chloro-o-xylene. What's more, it is a kind of clear colourless to light yellow liquid and belongs to the classes of Aromatic Halides (substituted); Halogen toluene; Chlorine Compounds. What's more, it should be stored in sealed container, and placed in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 3.73; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.73; (4)ACD/LogD (pH 7.4): 3.73; (5)ACD/BCF (pH 5.5): 405.01; (6)ACD/BCF (pH 7.4): 405.01; (7)ACD/KOC (pH 5.5): 2558.75; (8)ACD/KOC (pH 7.4): 2558.75; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 0 Å2; (13)Index of Refraction: 1.521; (14)Molar Refractivity: 40.79 cm3; (15)Molar Volume: 133.9 cm3; (16)Polarizability: 16.17×10-24cm3; (17)Surface Tension: 31.9 dyne/cm; (18)Enthalpy of Vaporization: 40.49 kJ/mol; (19)Vapour Pressure: 0.931 mmHg at 25°C.
Preparation of Benzene,1-chloro-2,3-dimethyl-: it can be prepared by 2,3-Dimethylphenyl-chlorameisensaeureester. This reaction will need reagent AlCl3 and various solvents. The reaction time is 210 minutes at reaction temperature of 200 °C. The yield is about 60%.
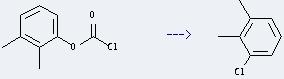
Uses of Benzene,1-chloro-2,3-dimethyl-: it can be used to get 3-chloro-phthalic acid. This reaction will need reagents cetyltrimethylammonium bromide and KMnO4, and solvent H2O. The reaction time is 24 hours at reaction temperature of 50-60 °C. The yield is about 40%.
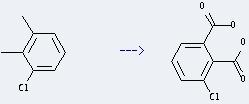
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes, respiratory system and skin. During using it, wear suitable protective clothing, gloves and eye/face protection. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: Clc1cccc(c1C)C
(2)InChI: InChI=1/C8H9Cl/c1-6-4-3-5-8(9)7(6)2/h3-5H,1-2H3
(3)InChIKey: NVLHGZIXTRYOKT-UHFFFAOYAS
Related Products
- 1-Chloro-2-(difluoromethyl)-3-fluorobenzene
- 1-Chloro-2-(methylsulphonyl)-4-nitrobenzene
- 1-CHLORO-2,2,5,5-TETRAMETHYL-4-IMIDAZOLIDINONE
- 1-Chloro-2,2-difluoropropane
- 1-Chloro-2,3-dimethylbenzene
- 1-Chloro-2,4-difluorobenzene
- 1-CHLORO-2,4-DIMETHOXY-5-NITROBENZENE
- 1-Chloro-2,4-dimethoxybenzene
- 1-Chloro-2,5-diethoxy-4-nitrobenzene
- 1-Chloro-2,5-difluorobenzene
- 608-25-3
- 6082-66-2
- 60827-45-4
- 608-27-5
- 608-28-6
- 608-30-0
- 608-31-1
- 60831-31-4
- 60832-72-6
- 608-33-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View